个人资料
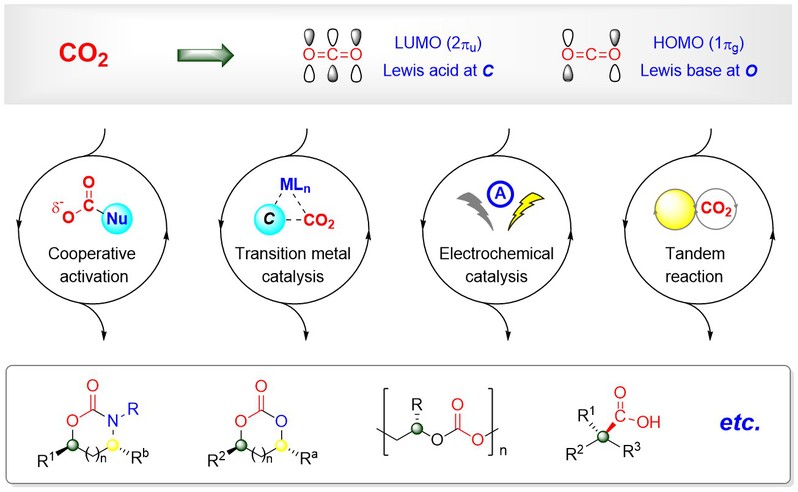
教育经历2005.9–2009.6, 四川师范大学, 材料化学, 学士 2009.9–2014.6, 华东师范大学, 有机化学, 博士, 导师: 周剑 2014.7–2017.12, 华东师范大学, 化学与分子工程学院, 讲师 2017.12–2021.12, 华东师范大学, 化学与分子工程学院, 副教授 2021.12–今, 华东师范大学, 化学与分子工程学院, 教授(破格晋升) 工作经历个人简介社会兼职中国化工学会硅能源与化工专委会副秘书长 《中国化学快报》青年编委会委员 《有机化学》青年编委会委员 研究方向主要围绕化学固定二氧化碳开展研究工作,探索二氧化碳参与的不对称催化新反应,通过开发更为高效的催化体系,基于电化学或不对称串联反应等策略,实现手性环碳酸酯或羧酸等系列高附加值化合物的高选择性合成。
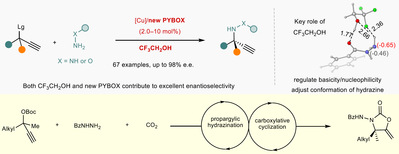
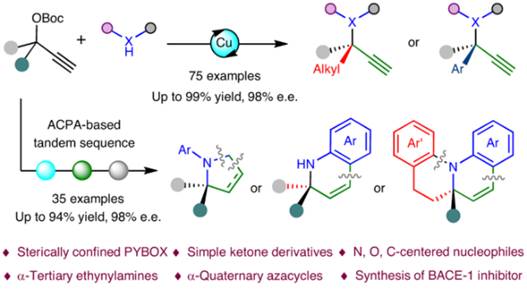
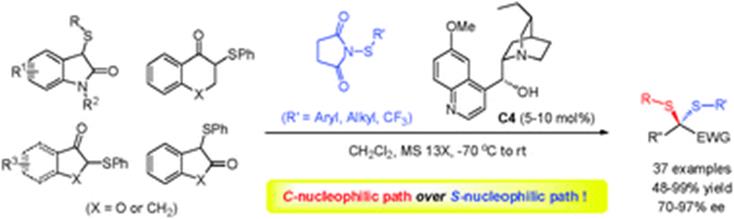
招生与培养开授课程本科生教学 2016年至今 担任本科生《有机化学实验》教学工作 2018年至今 开设本科生选修课程《化学中的人类文明》 2019年至今 担任本科生基础必修课程《有机化学》教学工作 研究生教学 2016年至今 担任《实验室安全与法律法规》研究生必修课程教学工作 2018年至今 担任《不对称催化》研究生专业选修课程教学工作 科研项目主持项目情况: 1. 国家重点研发计划“环境友好的土壤、粮食熏蒸处理新技术及产品研发”子课题负责人,2023 2. 国家自然科学基金面上项目,22171090,基于电化学的碳氢键或碳氟键与二氧化碳的羧化反应研究,2021 3. 国家自然科学基金面上项目,21871090,二氧化碳作为 C1 合成子的不对称催化反应研究,2018 4. 国家自然科学基金青年基金项目,21502053,手性硫缩醛/酮的不对称催化合成研究,2015 5. 巴斯夫新材料有限公司横向合作项目,2021 6. 巴斯夫新材料有限公司横向合作项目,2020 学术成果49. Yi Gong, Zheng Zhang, Huijuan Liu, Tao Wang, Mengmeng Jiang, Nan Feng, Peiying Peng, Huimin Wang, Feng Zhou*, Xin Wang* and Jian Zhou*, Trifluoroethanol-assisted asymmetric propargylic hydrazination to α-tertiary ethynylhydrazines enabled by sterically confined pyridinebisoxazolines.Nat. Commun.2025, https://doi.org/10.1038/s41467-025-59845-5
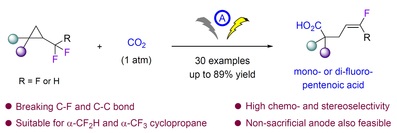
48. Nan Feng, Mengmeng Jiang, Huimin Wang, Yuqing Zhong, Ying Sun, Deyong Yang and Feng Zhou*Electrochemical carboxylation of α-fluoroalkyl cyclopropane with CO2 to mono- or difluoropentenoic acid. Org. Chem. Front.2025, 10.1039/d5qo00223k.
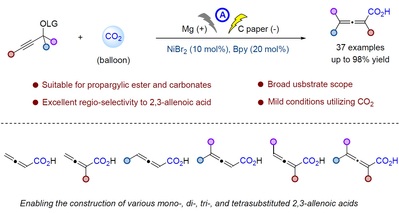
47. Yuqing Zhong, Mengmeng Jiang, Deyong Yang, Nan Feng, Ying Sun, Huimin Wang, Feng Zhou*, Nickel-catalyzed electrochemical carboxylation of propargylic esters with CO2 to 2,3-allenoic acids. Chin. Chem. Lett.2025, 111169.
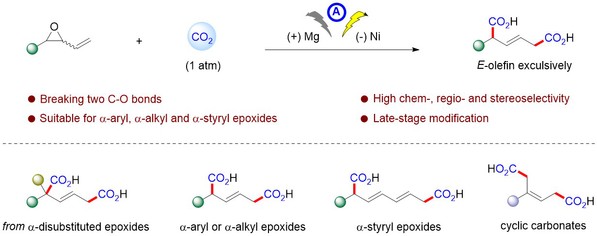
46. Deyong Yang, Ying Sun, Nan Feng, Yuqing Zhong, Jian Zhou and Feng Zhou*, Electrochemical Dicarboxylation of Vinyl Epoxide with CO2 for the Facile and Selective Synthesis of Diacids. Angew. Chem. Int. Ed. 2025, e202419702.
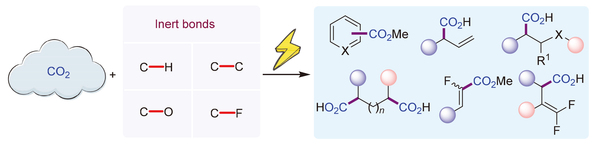
45. 高小童*,钟昱卿,冯楠,孙莹,杨得勇,周锋*,惰性键与二氧化碳的电化学羧化反应 研究(封面文章),《有机化学》2024, 44, 3043-3062.《有机化学》“C1化学专辑”邀请综述。
45. Zheng Zhang†, Zhi-Hao Zhang†, Ying Sun, Yun-Hao Tang, Yi-Zhuo Yang, Feng Zhou*, Jian Zhou. Tandem asymmetric propargylic amination/carboxylative cyclization reaction to chiral 5-methylidene-2-oxazolidinones using CO2 as C1 synthon. Sci. China Chem.2025, 68, 1402-1411.
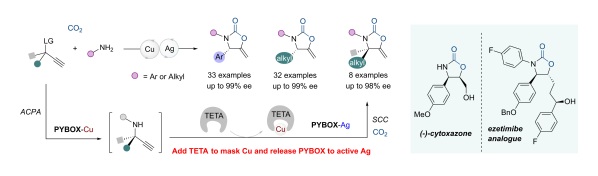
44. Z. Zhang, Y. Sun, Y. Gong, D. Tang, H. Luo, Z. Zhao, F. Zhou,* X. Wang* and J. Zhou* Enantioselective propargylic amination and related tandem sequences to α-tertiary ethynylamines and azacycles. Nat. Chem. 2024, 16, 521-532. (Highlight by Nat. Chem. 2024, 16, 483-484; Sci. China Chem. 2024, 67, 3181-3183; Synform, 2024, A143-A146; Synfacts, 2024, 20, 0608; Chin. J. Org. Chem. 2024, 44, 1699-1700)
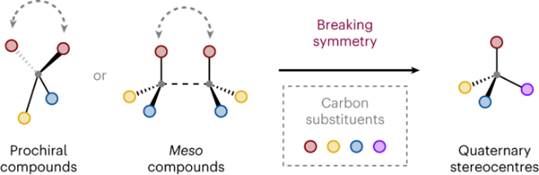
43. P.-W. Xu, F. Zhou,* Lei Zhu* and J. Zhou*,Catalytic desymmetrization reactions to synthesize all-carbon quaternary stereocentres. Nat. Synth. 2023, 2, 1020–1036.
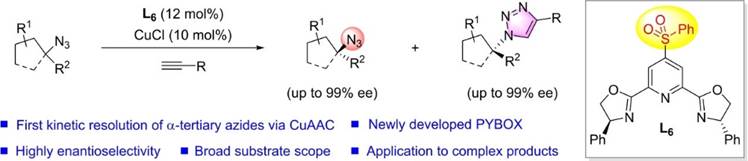
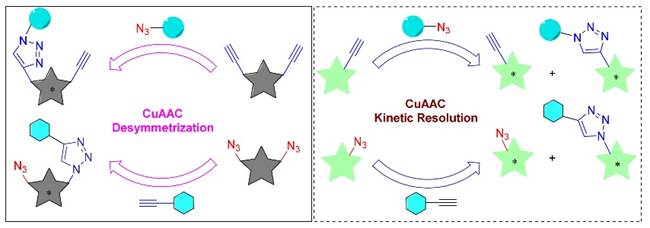
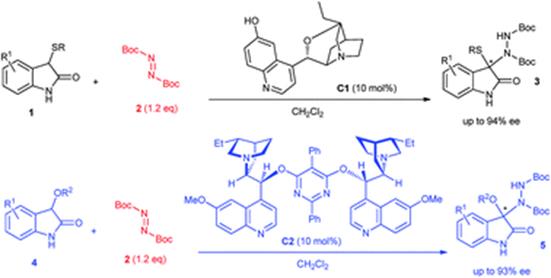
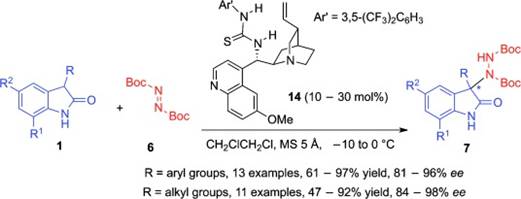
42. Y. Gong, C. Wang, F. Zhou,* K. Liao, X.-Y. Wang, Y. Sun, Y.-X. Zhang, Z. Tu, X. Wang,* and J. Zhou*, Sulfonyl-PYBOX Ligands Enable Kinetic Resolution of α-Tertiary Azides by CuAAC. Angew. Chem. Int. Ed. 2023, 62, e202301470.
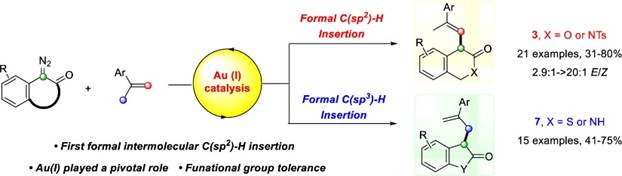
41. X.-Y. Cui, Z.-T. Ye, H.-H. Wu,* C.-G. Ji,* F. Zhou* and J. Zhou, Au(I)-Catalyzed Formal Intermolecular Carbene Insertion into Vinylic C(sp2)−H Bonds and Allylic C(sp3)−H Bonds. ACS Catal. 2023, 13, 1554−1561.
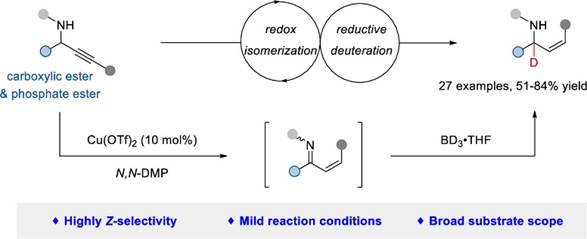
40. Z.-P. Zhao, H.-R. Lin, Z. Zhang, X.-T. Gao, C.-B. Ji,* J. Zhou, and F. Zhou*, A Highly Stereoselective Redox Isomerization-Reductive Deuteration Sequence of Propargyl Amines to α‑Deuterated Amino Acids. Org. Lett. 2023, 25, 7895−7899.
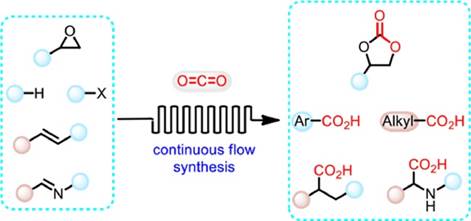
39. H. Luo, J. Ren, Y. Sun, Y.-L. Liu, F. Zhou,* G.-Y. Shi,* and Jian Zhou, Recent advances in chemical fixation of CO2 based on flow chemistry. Chin. Chem. Lett. 2023, 34, 107782.
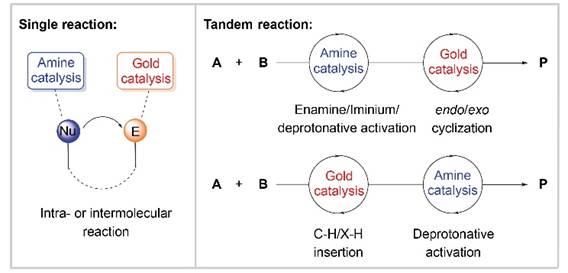
38. X.-Y. Cui, F. Zhou,* H.-H. Wu* and J. Zhou*, Asymmetric Tandem Reactions Achieved by Chiral Amine & Gold(I) Cooperative Catalysis. Chin. J. Org. Chem. 2022, 42, 3033-3050.
37. B.-W. Pan, Y. Shi, S.-Z. Dong, J.-X. He, B.-S. Mu, W.-B. Wu, Y. Zhou,* F. Zhou* and J. Zhou*, Highly stereoselective synthesis of spirocyclopropylthiooxindoles and biological evaluation. Org. Chem. Front. 2022, 9, 2640–2646.
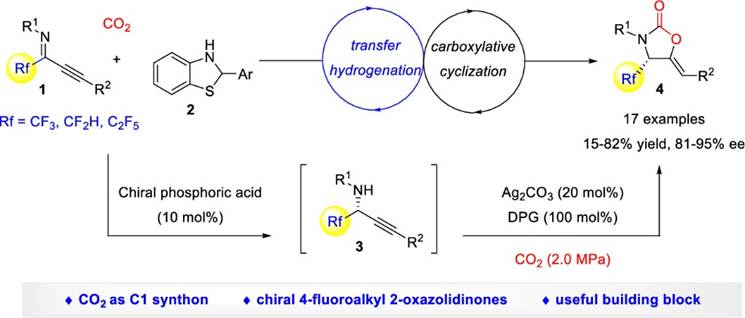
36. Z. Zhang, Z.-H. Zhang, F. Zhou,* and Jian Zhou, Catalytic Enantioselective Transfer Hydrogenation−Carboxylative Cyclization to 4‑Fluoroalkyl 2‑Oxazolidinone with CO2 as the C1 Synthon. Org. Lett. 2021, 23, 2726−2730.
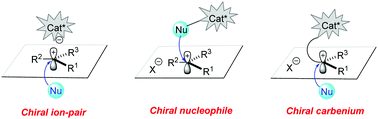
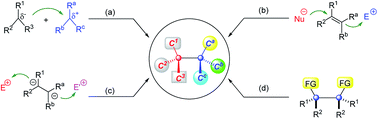
35. C.-W. Lei, B.-S. Mu, F. Zhou,* J.-S. Yu,* Y. Zhou* and J. Zhou, Organocatalytic enantioselective reactions involving prochiral carbocationic intermediates. Chem. Commun. 2021, 57, 9178.
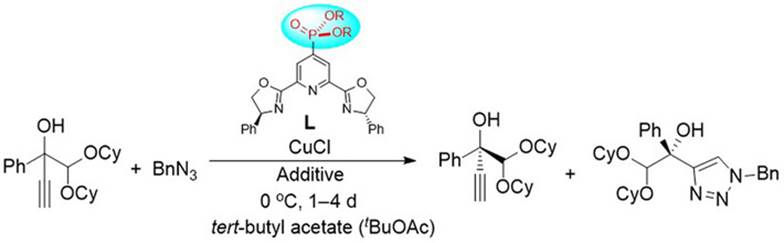
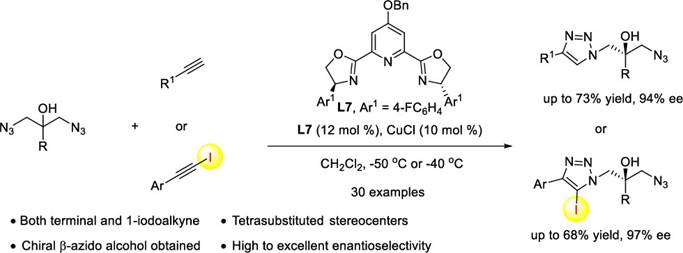
34. K. Liao, Y. Gong, R.-Y. Zhu, C. Wang, F. Zhou* and J. Zhou* Highly Enantioselective CuAAC of Functional Tertiary Alcohols Featuring an Ethynyl Group and Their Kinetic Resolution. Angew. Chem. Int. Ed. 2021, 60, 8488–8493.
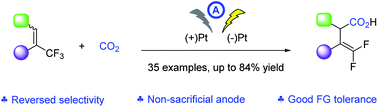
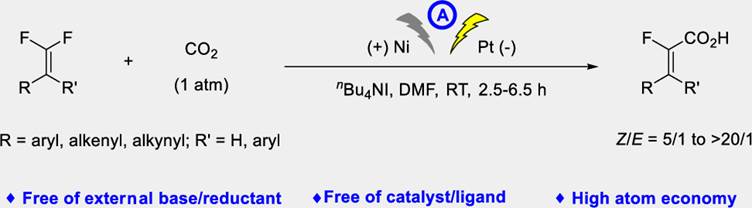
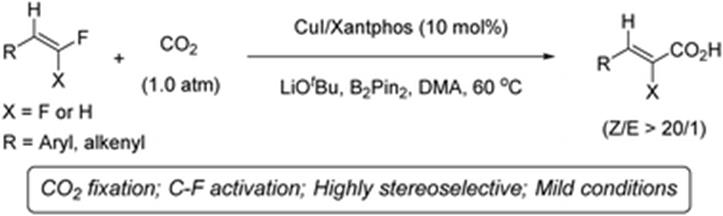
33. X.-T. Gao, Z. Zhang, X. Wang, J.-S. Tian, S.-L. Xie, F. Zhou* and J. Zhou, Direct electrochemical defluorinative carboxylation of alpha-CF3 alkenes with carbon dioxide. Chem. Sci. 2020, 11, 10414-10420.
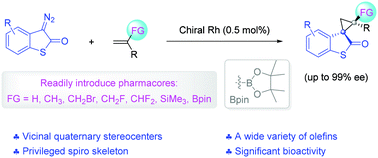
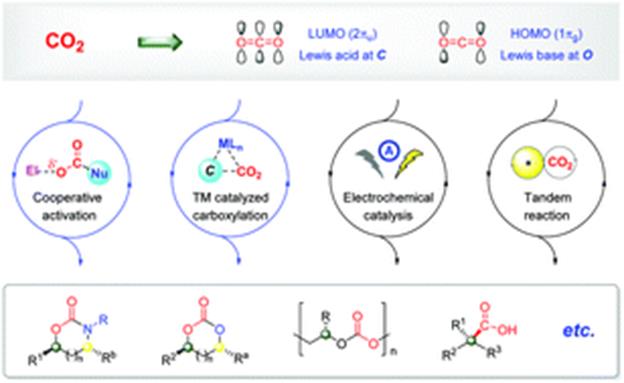
32. F. Zhou,* L. Zhu,* B.-W. Pan, Y. Shi, Y.-L. Liu and J. Zhou* Catalytic enantioselective construction of vicinal quaternary carbon stereocenters. Chem. Sci. 2020, 11, 9341-9365.
31. S.-L. Xie, X.-T. Gao, H.-H. Wu,* F. Zhou,* and J. Zhou, Direct Electrochemical Defluorinative Carboxylation of gemDifluoroalkenes with Carbon Dioxide. Org. Lett. 2020, 22, 8424-8429.
30. C. Wang, R.-Y. Zhu, K. Liao, F. Zhou,* and J. Zhou*, Enantioselective Cu(I)-Catalyzed Cycloaddition of Prochiral Diazides with Terminal or 1-Iodoalkynes, Org. Lett. 2020, 22, 1270-1274.
29. C. Wang, F. Zhou,* J. Zhou,* Recent Advances in the Enantioselective Copper(I)-Catalyzed Azide-Alkyne Cycloaddition Reaction. Chin. J. Org. Chem. 2020, 40, 3065-3077.
28. Y. Shi, B.-W. Pan, Y. Zhou,* J. Zhou, Y.-L. Liu and F. Zhou* Catalytic enantioselective synthesis using carbon dioxide as a C1 synthon. Org. Biomol. Chem. 2020, 18, 8597-8619.
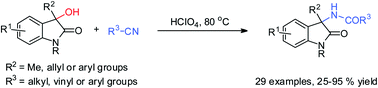
27. S. Xie, X. Gao, F. Zhou*, H. Wu*, J. Zhou*, Enantioselective carboxylative cyclization of propargylic alcohol with carbon dioxide under mild conditions. Chin. Chem. Lett. 2020, 31, 324-328.
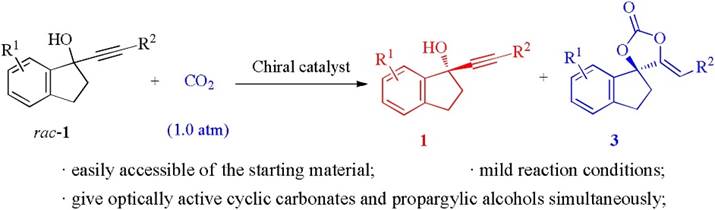
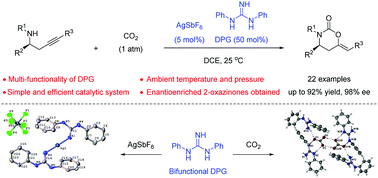
26. X.-T. Gao, S.-L. Xie, F. Zhou*, H.-H. Wu*, J. Zhou*, Multifunctional 1,3-diphenylguanidine for the carboxylative cyclization of homopropargyl amines with CO2 under ambient temperature and pressure.Chem. Commun. 2019, 55, 14303-14306.
25. S.-L. Xie, X.-Y. Cui, X.-T. Gao, F. Zhou*, H.-H. Wu*, J. Zhou, Stereoselective defluorinative carboxylation of gem-difluoroalkenes with carbon dioxide. Org. Chem. Front. 2019, 6, 3678-3682.
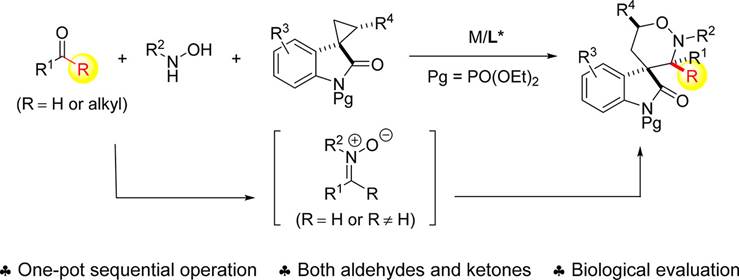
24. P.-W. Xu,C. Chen,J.-K. Liu,Y.-T. Song,F. Zhou*, J. Yan*, J. Zhou*, One-Pot Sequential [3 + 3] Dipolar Cycloaddition of Aldehyde or Ketone and Hydroxylamine with Spirocyclopropyl Oxindole. J. Org. Chem. 2018, 83, 12763−12774.
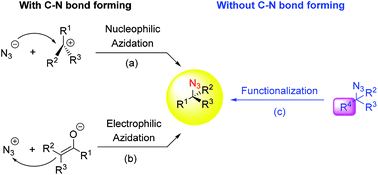
23. P.-G. Ding, X.-S. Hu,F. Zhou*, J. Zhou*, Catalytic enantioselective synthesis of α-chiral azides. Org. Chem. Front. 2018, 5, 1542-1559.
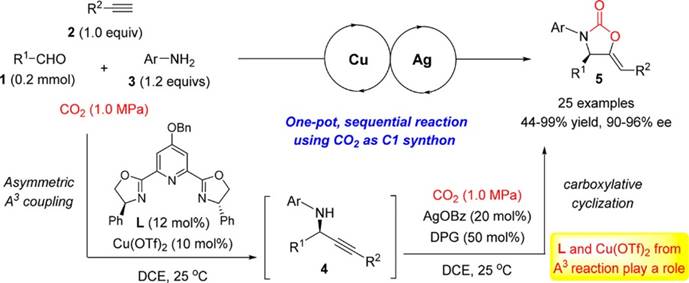
22. X.-T. Gao, C.-C. Gan, S.-Y. Liu, F. Zhou*, H.-H. Wu*, J. Zhou*, Utilization of CO2 as a C1 Building Block in a Tandem Asymmetric A3 Coupling-Carboxylative Cyclization Sequence to 2‑Oxazolidinones. ACS Catal. 2017, 7, 8588-8593.
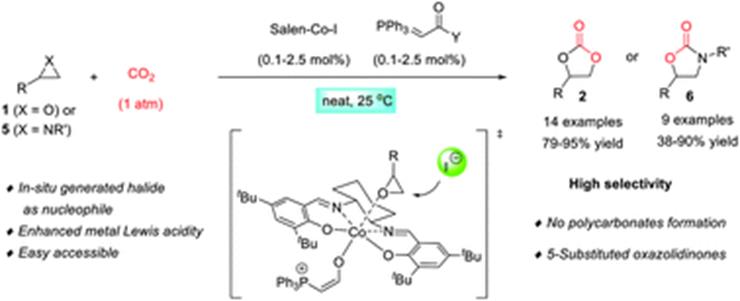
21. F. Zhou*, S.-L. Xie, X.-T. Gao, R. Zhang,* C.-H. Wang, G.-Q. Yin, J. Zhou* Activation of (salen)CoI complex by phosphorene for carbon dioxide transformation at ambient temperature and pressure. Green Chem. 2017, 19, 3908-3915.
20. J.-S. Yu, W.-B. Wu, F. Zhou*, The first catalytic asymmetric thioacetalization by chiral phosphoric acid catalysis, Org. Biomol. Chem. 2016, 14, 2205-2209.
19. K. Liao, F. Zhou*, J.-S. Yu, W.-M. Gao, J. Zhou, Catalytic asymmetric sulfenylation to structurally diverse dithioketals, Chem. Commun. 2015, 51, 16255-16258.
18. Z.-Y. Cao, W. D. G. Brittain, J. S. Fossey, F. Zhou* Recent advances in the use of chiral metal complexes with achiral ligands for application in asymmetric catalysis. Catal. Sci. Technol. 2015, 5, 3441-3451
研究生期间发表论文 17. F. Zhou, F.-M. Liao, J.-S. Yu, J. Zhou,* Catalytic Asymmetric Electrophilic Amination Reactions To Form Nitrogen-Bearing Tetrasubstituted Carbon Stereocenters. Synthesis, 2014, 46, 2983-3003.
16. F. Zhou, C. Tan, J. Tang, Y.-Y. Zhang, W.-M. Gao, H.-H. Wu, Y.-H. Yu and J. Zhou,*Asymmetric Copper(I)-Catalyzed Azide–Alkyne Cycloaddition to Quaternary Oxindoles. J. Am. Chem. Soc. 2013‚ 135‚ 10994-10997
15. F. Zhou, X.-P. Zeng, C. Wang, X.-L. Zhao and J. Zhou*. Organocatalytic asymmetric synthesis of 3,3-disubstituted oxindoles featuring two heteroatoms at the C3 position. Chem. Commun., 2013, 49, 2022.
14. F. Zhou‚ M. Ding‚ J. Zhou,*A Catalytic Metal-free Ritter Reaction to 3-Substituted 3-Aminooxindoles. Org. Biomol. Chem. 2012‚ 10‚ 3178-3183.
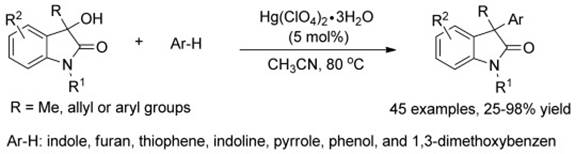
13. F. Zhou‚ Z.-Y. Cao‚ J. Zhang‚ H. B. Yang‚ J. Zhou,*A Highly Efficient Friedel–Crafts Reaction of 3-Hydroxyoxindoles and Aromatic Compounds to 3‚3-Diaryl and 3-Alkyl-3-aryloxindoles Catalyzed by Hg(ClO4)2⋅3H2O. Chem. Asian J. 2012‚ 7‚ 233-241.
12. F. Zhou‚ M. Ding‚ Y.-L. Liu‚ C.-H. Wang‚ C.-B. Ji‚ Y.-Y. Zhang, J. Zhou,*Organocatalytic Asymmetric α-Amination of Unprotected 3-Aryl and 3-Aliphatic Substituted Oxindoles using Di-tert-butyl Azodicarboxylate Adv. Syn. Catal. 2011‚ 353‚ 2945-2952.
11. F. Zhou‚ Y.-L. Liu‚ J. Zhou‚*Catalytic Asymmetric Synthesis of Oxindoles Bearing a Tetrasubstituted Stereocenter at the C-3 Position. Adv. Syn. Catal. 2010‚ 352‚ 1381-1407.
10. Z.-Y. Cao, F. Zhou, Y.-H. Yu, J. Zhou,*A Highly Diastereo- and Enantioselective Hg(II)-Catalyzed Cyclopropanation of Diazooxindoles and Alkenes Org. Lett.2013‚ 15, 42-45. 9. F. Zhu, F. Zhou, Z.-Y. Cao, C. Wang, Y.-X. Zhang, C.-H. Wang*, J. Zhou,*A Facile Method for the Synthesis of 3-Substituted 3-(Alkylthio)oxindoles or 3-Alkoxyoxindoles Synthesis, 2012, 44, 3129-3144. 8. J.-S. Yu, F. Zhou, Y.-L. Liu, J. Zhou*, Organocatalytic asymmetric Michael addition of unprotected 3-substituted oxindoles to 1,4-naphthoquinone. Beilstein. J. Org. Chem. 2012, 8, 1360-1365. 7. L. Chen‚ F. Zhou‚ T.-D. Shi‚ J. Zhou*,Metal-Free Tandem Friedel–Crafts/Lactonization Reaction to Benzofuranones Bearing a Quaternary Center at C3 Position J. Org. Chem. 2012‚ 77‚ 4354-4362. 6. M. Ding‚ F. Zhou‚ Y.-L. Liu‚ C.-H. Wang‚ X.-L. Zhao‚ J. Zhou,* Cinchona alkaloid-based phosphoramide catalyzed highly enantioselective Michael addition of unprotected 3-substituted oxindoles to nitroolefinsChem. Sci. 2011‚ 2‚ 2035-2039. 5. Y.-L. Liu‚ T.-D. Shi‚ F. Zhou‚ X.-L. Zhao‚ X. Wang‚* J. Zhou,*Organocatalytic Asymmetric Strecker Reaction of Di- and Trifluoromethyl Ketoimines. Remarkable Fluorine Effect Org. Lett. 2011‚ 13‚ 3826-3829. 4. Y.-L. Liu‚ F. Zhou‚ J.-J. Cao‚ C.-B. Ji‚ M. Ding‚ J. Zhou,*A facile method for the synthesis of oxindole based quaternary α-aminonitriles via the Strecker reactionOrg. Biomol. Chem. 2010‚ 8‚ 3847-3850. 3. J.-J. Cao‚ F. Zhou‚J. Zhou‚*Improving the Atom Efficiency of the Wittig Reaction by a “Waste as Catalyst/Co-catalyst” Strategy Angew. Chem. Int. Ed. 2010‚ 49‚ 4976-4980. 2. M. Ding‚ F. Zhou‚ Z.-Q. Qian‚ J. Zhou‚*Organocatalytic Michael addition of unprotected 3-substituted oxindoles to nitroolefins. Org. Biomol. Chem. 2010‚ 8‚ 2912-1914. 1. Z.-Q. Qian‚ F. Zhou‚ T.-P. Du‚ M. Ding‚ B.-L. Wang‚ X.-L. Zhao‚ J. Zhou‚*Asymmetric construction of quaternary stereocenters by direct organocatalytic amination of 3-substituted oxindolesChem. Commun. 2009‚ 6753-6755.
荣誉及奖励2016年6月,上海市优秀博士学位论文 2014年5月,上海市优秀毕业生 2013年12月,华东师范校长奖学金 2013年12月,博士研究生国家奖学金 2012年12月,华东师范大学校长奖学金一等奖 |

|
周锋 |