About
EducationWorkExperienceResumeOther AppointmentsResearch Fields(a) Organo Metallics (b)Metal-catalyzed novel mothodologies towards pharmaceuticaes Enrollment and TrainingCourseScientific ResearchAcademic Achievements
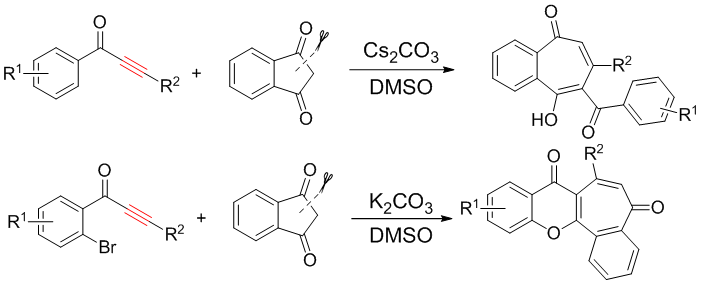
[17] Transition-metal-free Ring Expansion Reactions of Indene-1,3-dione: Synthesis of Functionalized Benzoannulated Seven-Membered Ring Compounds,Qiyi Yao, Lingkai Kong, Mengdan Wang, Yang Yuan, Ruizhuo Sun,Yanzhong Li* Org. Lett., DOI: 10.1021/acs.orglett.8b00206
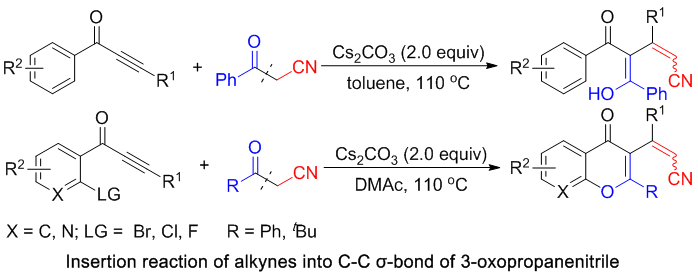
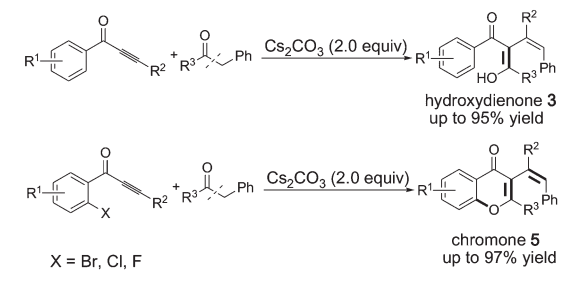
[16] Base-Promoted Tandem Reaction towards Conjugated Dienone or Chromone Derivatives with a Cyano Group: Insertion of Alkynes into C–C σ-Bonds of 3-Oxopropanenitriles, Qiyi Yao, Lingkai Kong, Fangfang Zhang, Xianghua Tao and Yanzhong Li*,Adv. Synth. Catal. 2017, 359, 3079–3084.
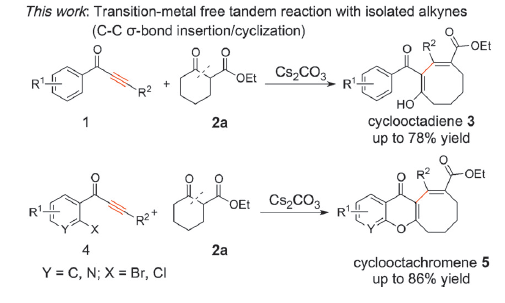
[15] Insertion of Isolated Alkynes into Carbon–Carbon s-Bonds of Unstrained Cyclic b-Ketoesters via Transition-Metal-Free Tandem Reactions: Synthesis of Medium-Sized Ring Compounds,Yuanyuan Zhou, Xianghua Tao, Qiyi Yao, Yulei Zhao, and Yanzhong Li*,Chem. Eur. J., 2016, 22, 17936 – 17939
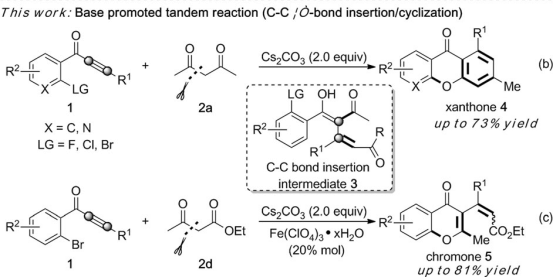
[14] Base-Promoted Tandem Reaction Involving Insertion into Carbon–Carbon s-Bonds: Synthesis of Xanthone and Chromone Derivatives,Xingcan Cheng, Yuanyuan Zhou, Fangfang Zhang, Kai Zhu, Yuanyuan Liu, and Yanzhong Li*,Chem. Eur. J., 2016, 22, 1 – 6
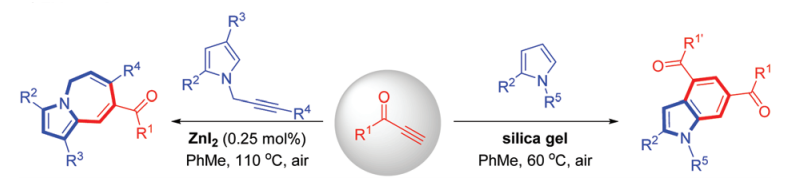
[12] Selective synthesis of pyrrolo[1,2-a]azepines or 4,6-dicarbonyl indoles via tandem reactions of alkynones with pyrrole derivatives, Yulei Zhao, Yang Yuan, Murong Xu, Zhong Zheng, Runhua Zhang and Yanzhong Li*, Org. Biomol. Chem., 2017, 15, 6328-6332.
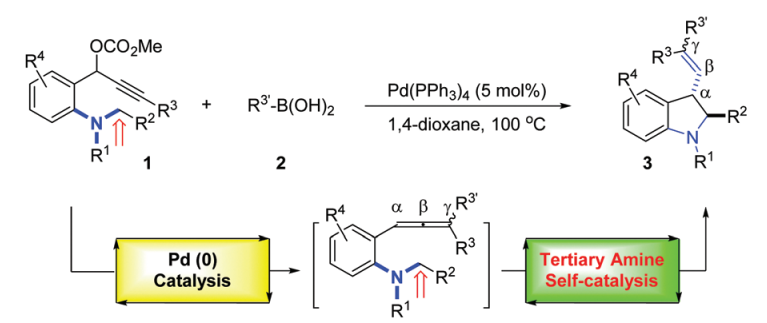
[11] Tertiary amine self-catalyzed intramolecular Csp3–H functionalization with in situ generated allenes for the formation of 3-alkenyl indolines,Yulei Zhao, Murong Xu, Zhong Zheng, Yang Yuan and Yanzhong Li*,Chem. Commun..2017, 53, 3721-3724.
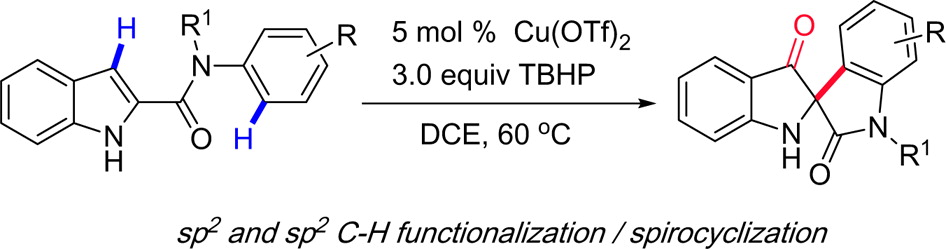
[10] Copper-Catalyzed Oxidative Dearomatization/Spirocyclization of Indole-2-Carboxamides: Synthesis of 2‑Spiro-pseudoindoxyls, Lingkai Kong, Mengdan Wang, Fangfang Zhang, Murong Xu, and Yanzhong Li*, Org. Lett., 2016, 18, 6124-6127.
[9] Metal/Benzoyl Peroxide (BPO)-Controlled Chemoselective Cycloisomerization of (o‑Alkynyl)phenyl Enaminones: Synthesis of α‑Naphthylamines and Indeno[1,2‑c]pyrrolones, Fangfang Zhang, Zhengchen Qin, Lingkai Kong, Yulei Zhao, Yuanyuan Liu, and Yanzhong Li*, Org. Lett.,2016, 18, 5150-5153.
[8] Synthesis of polycyclic benzo[b]indolo[3,2,1-de]acridines via sequential allenylation/Diels-Alder cyclization/hydrogen migration reaction, Yulei Zhao, Yang Yuan, Xiaoyu Wang and Yanzhong Li*, J. Org. Chem., 2017, 82,11198–11205.
[7] Synthesis of 1-Alkyl-3-(2-oxo-2-aryl/alkyl-ethyl)indolin-2-ones through Gold/Brønsted Acid Relay Actions: Observation of Selective C=C Bond Cleavage of Enaminones, Yulei Zhao, Yang Yuan, Lingkai Kong, Fangfang Zhang and Yanzhong Li*, Synthesis, 2017, 49, 3609-3618.
[6] Pd-Catalyzed C-H/N-H Arylation: One-Pot Synthesis of Indolo[1,2-f]phenanthridines, Lingkai Kong, Qiyi Yao, Mengdan Wang, Ruizhuo Sun and Yanzhong Li *, ChemSelect, 2018, 3, 456–460.
[5] Insertion of Isolated Alkynes into Carbon–Carbon s-Bonds of Unstrained Cyclic b-Ketoesters via Transition-Metal-Free Tandem Reactions: Synthesis of Medium-Sized Ring Compounds, Yuanyuan Zhou, Xianghua Tao, Qiyi Yao, Yulei Zhao, and Yanzhong Li*,Chem. Eur. J., 2016, 22, 17936–17939.
[4] Base-Promoted Tandem Reaction Involving Insertion into Carbon– Carbon s-Bonds: Synthesis of Xanthone and Chromone Derivatives, X. Cheng, Y. Zhou, F. Zhang, K. Zhu, Y. Liu and Y. Li*, Chem. Eur. J., 2016, 22, 12655–12659.
[3] Gold-catalyzed chemo- and diastereoselective C(sp2)–H functionalization of enaminones for the synthesis of pyrrolo[3,4-c]-quinolin-1-one derivatives,Y. Zhao, Q. Duan, Y. Zhou, Q. Yao and Y. Li*,Org. Biomol. Chem., 2016, 14, 2177-2181.
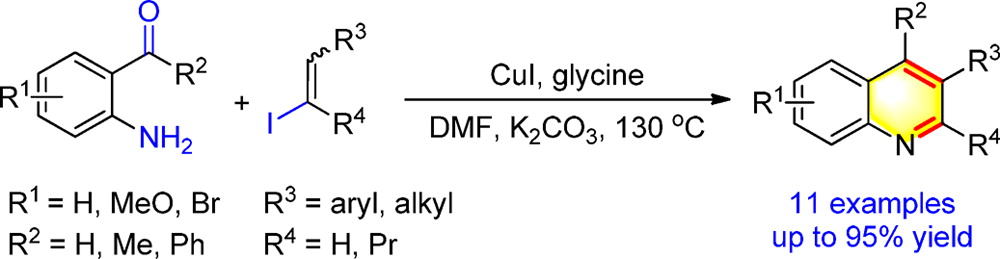
[2] Copper-Catalyzed Synthesis of Substituted Quinolines via C-N Coupling/Condensation from ortho-Acylanilines and Alkenyl Iodides,L. Kong, Y. Zhou, H. Huang, Y. Yang, Y. Liu, Y. Li*, J. Org. Chem., 2015, 80, 1275.
Honor |

|
Liyanzhong |