个人资料
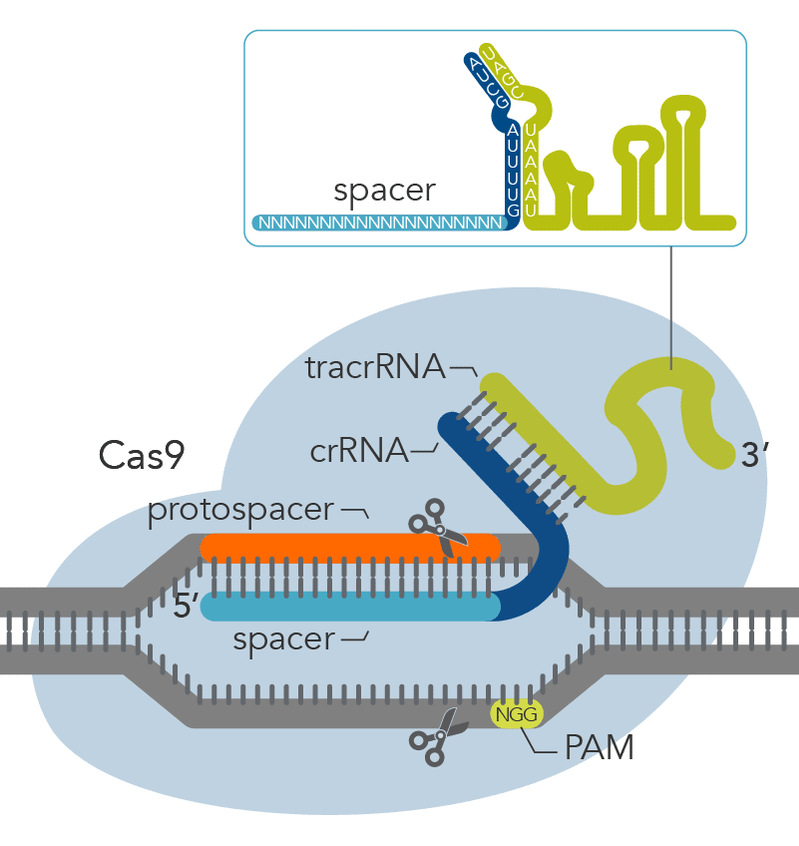
教育经历1995年7月-2000年8月:哈佛大学医学院,麻省理工学院 博士后 1990年1月-1995年6月:美国纽约州立大学,冷泉港研究所 博士 1987年7月-1989年12月:复旦大学医学院(原上海医科大学) 硕士 1982年9月-1987年6月:复旦大学医学院(原上海医科大学) 本科 工作经历个人简介钟涛教授主要以斑马鱼和小鼠为模式动物,结合人类干细胞,利用遗传学、发育生物学、化学生物学和分子生物学的方法研究心脏和血管的分化形成与再生的生物学机制。在国际科学界,第一次提出动脉血管内皮细胞在血液循环之前就已获得分子特征的概念,发现和克隆心血管发育的核心转录因子Grl/Hey2家族及其上游关键信号NOTCH通路。率先揭示了前列腺素(PGE2)信号及其转运蛋白(ABCC)调控细胞纤毛生长与心脏及多种器官形态发育的新颖生物学机制。以第一作者或通讯作者在包括Cell,Nature, Science, Nature Cell Biology等国际一流学术杂志上发表了80多篇文章。关于血管内皮细胞分化的研究成果被美国大学经典教科书《发育生物学》(第十版)采用(教科书级别重大成果)。 社会兼职研究方向研究成果和方向 1)利用基因编辑工具CRISPR/Cas9构建转基因、突变体斑马鱼以及开发更高效的单碱基编辑工具。 细胞谱系示踪是一种研究器官发育、组织损伤以及单细胞分化命运的重要手段,诱导性重组酶Cre/Loxp系统的应用为细胞谱系示踪技术打开了突破口。我们团队应用CRISPR/Cas9基因编辑工具(见图1)将外源性荧光报告基团序列或CreERT2序列定点整合到目的基因下游:①通过在斑马鱼活体中实时观察荧光基团表达位置及强度,可以反映目的基因内源性表达位置及表达丰度,以追踪表达此基因的细胞谱系;②通过控制外源诱导剂的时间窗口,诱导组织特异性表达的CreERT2对Loxp序列进行切割,以实现基因条件性敲除。同时,我们团队利用优化的高效单碱基编辑工具在斑马鱼当中进行基因编辑,构建斑马鱼疾病模型以模拟人类由于单碱基突变导致的疾病,为探索和研究人类单碱基突变疾病的病理机制及治疗方法奠定基础。基因碱基编辑器主要包括腺嘌呤碱基编辑器(ABE)与胞嘧啶碱基编辑器(CBE),可以在不产生DNA 双链断裂的情况下,在编辑窗口之内分别实现A:T>G:C 或者C:G>T:A 的转换,是基因编辑领域的颠覆性技术。我们团队合作开发了具有超高活性的腺嘌呤单碱基编辑器(hyABE),极大地提高了靠近PAM区A-to-G的转换效率,扩大了编辑窗口;研发的超高活性嘌呤嘧啶双碱基编辑器(eA&C-BEs和hyA&C-BEs)大幅提高了A-to-G和C-to-T(A/C)同时的编辑效率。研究证明hyABE在斑马鱼胚胎中可以有效催化A-to-G转换,编辑效率高达60%。hyABE能够在F0胚胎中引入纯合rps14E12G致病点突变,显著降低血红蛋白和红细胞数目,与人类血细胞发育异常(MDS)疾病表型一致,展现了hyABE在构建点突变疾病模型中的优势。同时,双碱基编辑器eA&C-BEs和hyA&C-BEs在斑马鱼胚胎中展现出较高的A-to-G和C-to-T同时转换效率。这些碱基编辑器的应用并没有引起斑马鱼胚胎发育致畸表型,提供了潜在的基因治疗新策略 (Nature Communications 2023) (https://www.ecnu.edu.cn/info/1094/62291.htm)。
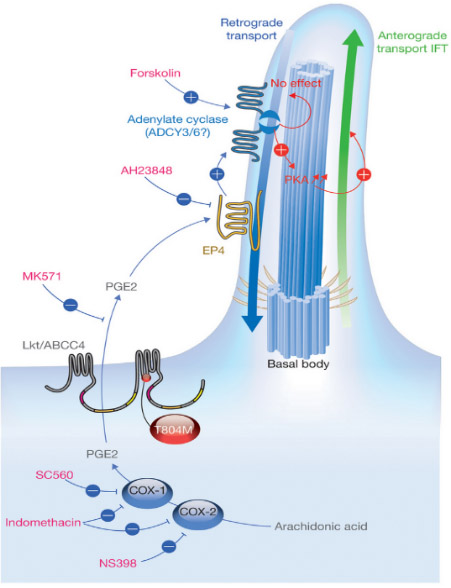
图1.CRISPR/Cas9基本结构示意图 2)细胞纤毛与胚胎及心脏生长发育机制研究 我们发现前列腺素信号通路调控细胞纤毛生长和心脏不对称发育 (Nature Cell Biology, 2014;封面长文)。研究团队以斑马鱼和人类细胞为模型,通过分析Leakytail (Lkt) 遗传突变体,发现前列腺素转运蛋白缺失造成心脏与其他内脏器官随机性偏侧等异常表型。证明这些异常表型主要是由于胚胎发育时期细胞表面纤毛生长缺陷所引起。研究小组进一步发现LKT转运蛋白能够从细胞内转运前列腺素到细胞外,后者通过结合定位于纤毛膜上的G蛋白偶联受体,进而影响纤毛内转运蛋白 (IFT) 的正向速度,最终调节纤毛生长和心脏及其他内脏器官不对称发育(见图2)。此发现不仅揭示了在胚胎形成和器官发育中前列腺素信号的重要性,而且有助于解密人类纤毛运动综合症和先天性心脏病的细胞分子病因。《自然·细胞生物学》发表专题评论,认为“这项研究工作是前列腺素生理信号在发育领域的一个意想不到的重大发现”。
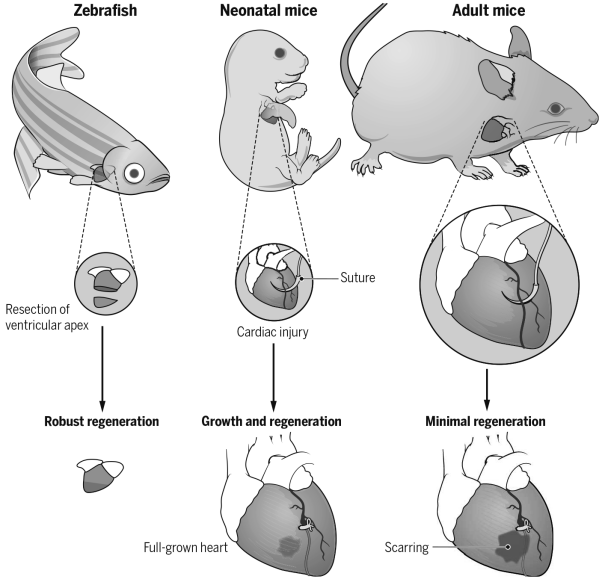
图2.PGE2信号通路调控纤毛生长 3)探索心脏发育及再生机制 成年哺乳动物心脏受损后再生能力有限,受损后心肌细胞被纤维疤痕取代,最终导致心力衰竭。与哺乳动物心脏不同,斑马鱼心脏损伤后可以通过心肌细胞去分化和增殖实现心脏再生;新生小鼠在出生一周内,损伤的心脏也可以通过心肌去分化和增殖完成再生。哺乳动物心脏再生能力有限,主要是由于细胞内在再生屏障与分子阻滞因子的存在,内源性再生障碍和负调控因子会阻碍心脏受损后心肌细胞去分化和增殖。探究这些再生过程中机制将为人类心脏疾病治疗提供新的启示。 我们实验室主要应用斑马鱼和小鼠作为心脏再生研究模型(见图3)。通过构建心肌特异性过表达T-box家族的转录因子(tbx20)品系,我们发现心肌细胞特异性过表达tbx20可以诱导受损区心肌细胞去分化,激活心内膜细胞BMP信号通路,促进受损心肌和心内膜再生(Frontiers in Cell and Developmental Biology 2020)。同时,我们揭示了bHLH转录因子Gridlock(Grl)/Hey2在心脏再生中的分子屏障机制;心肌细胞中条件性诱导Grl表达能够抑制受损后心肌细胞去分化与增殖;反之,Grl突变减少纤维化疤痕和促进心肌细胞增殖,增强心脏再生。研究表明心脏损伤诱导Grl表达下降,引起甲基转移酶Smyd2表达升高,进而调节Stat3甲基化和磷酸化,促进受损心肌去分化和再生(Development 2020)。
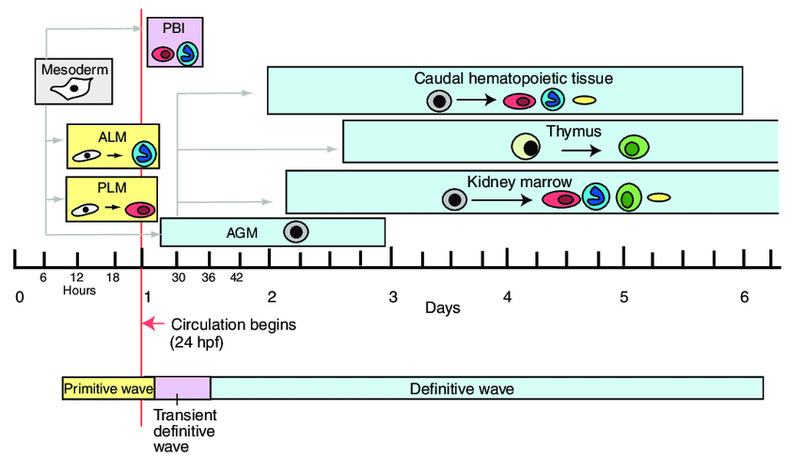
图3.心脏再生研究中常用的模式动物 4)研究造血干细胞增殖和分化的分子及细胞机制 造血干细胞(Hematopoietic and progenitor cells, HSPCs)是一群具有增殖、分化和重建能力的异质性干祖细胞。在血液形成过程中,造血干细胞是从主动脉-性腺-中肾区(Aorta-Gonad-Mesoneph, AGM)动脉腹侧生血内皮中产生,随后迁移至哺乳动物的胎肝(Feter Liver)或斑马鱼的尾部造血组织(Caudal Hematopoietic Tissue)进行快速增殖和分化,最终定植于骨髓或肾脏,维持成体造血(见图4)。我们研究团队以斑马鱼和小鼠为模式生物,结合人类造血干细胞,应用发育生物学、表观遗传学及基因组学方法,阐明了ATF7IP/SETDB1介导的组蛋白甲基化修饰是协调HSPC干细胞状态与多种血液谱系分化的一个关键检验点(Checkpoint),用于维持红系、髓系和淋巴系等多种细胞的平衡分化及血液系统的正常循环与功能。机制上,atf7ip或setdb1缺失造成细胞周期负调节因子cdkn1a/p21与髓系转录激活因子cebpß启动子区域H3K9me3沉积减少,染色质开放程度增加,导致cdkn1a/p21和cebpß表达增加,引起造血干细胞扩增受阻,红系分化减少,最终促进髓系分化偏倚。同时,Atf7ip与Setdb1相互作用,沉默逆转录转座子(LTR/LINE)表达,抑制Mda5/Rig-I介导的天然免疫应答以及应急引起的髓系偏倚分化。该研究揭示了ATF7IP/SETDB1介导的H3K9me3沉积和染色质重塑在控制造血干细胞扩增与多种血细胞分化中的一个重要调控机制,为急性髓系白血病及其他人类疾病的干预提供了新策略 (PNAS 2022) (https://www.ecnu.edu.cn/info/1094/61875.htm)。DHX15是一种RNA解旋酶,临床发现AML患者存在DHX15 R222G突变,与AML患者不良预后相关。研究团队发现dhx15 r222g突变体呈现HSPC增殖受阻的表型,证明HSPC缺陷是通过未折叠蛋白应答(UPR)通路介导,对评估AML发生和预后具有一定临床意义(Cancer Science 2021)。
图4.斑马鱼造血过程示意图 招生与培养开授课程秋季学期开授专业选修课《发育与疾病》。 科研项目研究团队 教授:钟涛 副教授:靳大庆、李东亮 博士后:佘培露、孙建建、胡雪丽 博士研究生:高邦君、朱雪娇、周亚婷、吴佳欣、李娟、刘旭、陈业伟 硕士研究生:贺渊、张琳、苗婷婷、强梦轲、仲祉霖、陈雯琪、万萌、史西亚、漆瑶、莫菲、张人杰 团队活动
“从波提切利到梵高-英国国家美术馆珍藏展”参观
黄果树瀑布游览
浦江夜游 学生荣誉
学术成果代表性论文 Xue N, Liu X, Zhang D, Wu Y, Zhong Y, Wang J, Fan W, Jiang H, Zhu B, Ge X, Gonzalez RVL, Chen L, Zhang S, She P, Zhong Z, Sun J, Chen X, Wang L, Gu Z, Zhu P, Liu M, Li D, Zhong TP*, Zhang X*. 2023. Improving adenine and dual base editors through introduction of TadA-8e and Rad51DBD. Nature Communications. 14(1):1224. *Correspondent author
Liu X, Yu T, Tan X, Jin D, Yang W, Zhang J, Dai L, He Z, Li D, Zhang Y, Liao S, Zhao J, Zhong TP*, Liu C*. 2023. Renal interstitial cells promote nephron regeneration by secreting prostaglandin E2. Elife. 12:e81438. *Correspondent author
Wu J, Li J, Chen K, Liu G, Zhou Y, Chen W, Zhu X, Ni TT, Zhang B, Jin D, Li D, Kang L, Wu Y, Zhu P, Xie P, Zhong TP. 2023. Atf7ip and Setdb1 interaction orchestrates the hematopoietic stem and progenitor cell state with diverse lineage differentiation. Proc Natl Acad Sci. 120(1):e2209062120.
Li D, Sun J, Zhong TP. 2022. Wnt Signaling in Heart Development and Regeneration. Curr Cardiol Rep. 24(10):1425-1438.
Jin D, Zhong TP. 2022. Prostaglandin signaling in ciliogenesis and development. J Cell Physiol. 237(6):2632-2643.
Liu, X.X., Pu, W.J., He, L.J., Li, Y., Zhao, H., Li, Y., Liu, K., Huang, X.Z., Weng, W.D., Wang, Q.D., Shen, L.H., Tao P. Zhong, Sun, K., Reza Ardehali, He, B., Zhou, B. 2021. Cell proliferation fate mapping reveals regional cardiomyocyte cell-cycle activity in subendocardial muscle of left ventricle. Nature Communications. 12(1):5784.
Cai Y, Wang J, Jin D, Liu Q, Chen X, Pan L, Li Y, Wang X, Qian F, Wang J, Zhong TP*, Wang S*. 2021. Dhx15 regulates zebrafish definitive hematopoiesis through the unfolded protein response pathway. Cancer Sci. 112(9):3884-3894. *Correspondent author
Wei G, Sun H, Dong K, Hu L, Wang Q, Zhuang Q, Zhu Y, Zhang X, Shao Y, Tang H, Li Z, Chen S, Lu J, Wang Y, Gan X, Zhong TP, Gui D, Hu X, Wang L, Liu J. 2021. The thermogenic activity of adjacent adipocytes fuels the progression of ccRCC and compromises anti-tumor therapeutic efficacy. Cell Metab. 33(10):2021-2039.e8.
Peilu She, Huifang Zhang, Xian.gwen Peng, Jianjian Sun, Bangjun Gao, Yating Zhou, Xuejiao Zhu, Xueli Hu, Kaa Seng Lai, Jiemin Wong, Bin Zhou, Linhui Wang, Tao P. Zhong. 2020. The Gridlock/Hey2 Transcriptional Repressor Impedes Vertebrate Heart Regeneration by Restricting Expression of Lysine Methyltransferase. Development. 147(18):dev190678.
Peng X, Lai KS, She P, Kang J, Wang T, Li G, Zhou Y, Sun J, Jin D, Xu X, Liao L, Liu J, Lee E, Poss KD, Zhong TP. 2021. Induction of Wnt signaling antagonists and p21-activated kinase enhances cardiomyocyte proliferation during zebrafish heart regeneration. J Mol Cell Biol. 13(1):41-58. Fang Y, Lai KS, She P, Sun J, Tao W, Zhong TP. 2020. Tbx20 Induction Promotes Zebrafish Heart Regeneration by Inducing Cardiomyocyte Dedifferentiation and Endocardial Expansion. Front Cell Dev Biol. 8:738.
Sun Y, Zhang B, Luo L, Shi DL, Wang H, Cui Z, Huang H, Cao Y, Shu X, Zhang W, Zhou J, Li Y, Du J, Zhao Q, Chen J, Zhong H, Zhong TP, Li L, Xiong JW, Peng J, Xiao W, Zhang J, Yao J, Yin Z, Mo X, Peng G, Zhu J, Chen Y, Zhou Y, Liu D, Pan W, Zhang Y, Ruan H, Liu F, Zhu Z, Meng A; ZAKOC Consortium. 2019. Systematic genome editing of the genes on zebrafish Chromosome 1 by CRISPR/Cas9. Genome Res. 30(1):118–26.
Zhao W, Cao L, Ying H, Zhang W, Li D, Zhu X, Xue W, Wu S, Cao M, Fu C, Qi H, Hao Y, Tang YC, Qin J, Zhong TP, Lin X, Yu L, Li X, Li L, Wu D, Pan W. 2019. Endothelial CDS2 deficiency causes VEGFA-mediated vascular regression and tumor inhibition. Cell Res. 29(11):895-910.
Xie S, Fu W, Yu G, Hu X, Lai KS, Peng X, Zhou Y, Zhu X, Christov P, Sawyer L, Ni TT, Sulikowski GA, Yang Z, Lee E, Zeng C, Wang WE, Zhong TP. 2019. Discovering small molecules as Wnt inhibitors that promote heart regeneration and injury repair. J Mol Cell Biol. 12(1):42-54
Li G, Jin D, Zhong TP. 2019. Tubgcp3 Is Required for Retinal Progenitor Cell Proliferation During Development. Frontier in Mol Neurosci. 12:126
Li W, Jin D, Zhong TP. 2019. Photoreceptor cell development requires prostaglandin signaling in the zebrafish retina. Biochem Biophys Res Commun.510(2):230-235
Zhang R, He J, Wang X, Pan W, Cao Y, Zu Y, Jiang Q, Du J, Zhong TP. 2019. Report of the Fifth Zebrafish Research Conference. Zebrafish.16(1):128-134.
Xinyu Yang, Jiani Li, Yabo Fang, Zhen Zhang, Daqing Jin, Xingdong Chen, YongtaoGuan, Yan Zhao, Mengqi Li, Linchun Huan, Thomas A. Kent, Jing-fei Dong, Rongcai Jiang, Shuyuan Yang, Li Jin, Jianning Zhang, Zhong TP+, Fuli Yu+. 2018. Rho Guanine Nucleotide Exchange Factor ARHGEF17 is a risk gene for Intracranial Aneurysms. Circulation: Genomic and Precision Medicine. 11(7):e002099. +Correspondent author
Daqing Jin, Diqi Zhu, Yabo Fang, Yiwei Chen, Gaihong Yu, Weijun Pan, Dong Liu, Fen Li, Zhong TP. 2017. Vegfa signaling regulates diverse artery/vein formation in vertebrate vasculatures. J Genet Genomics. 44(10):483-492
Xueying Tian, Yan Li, Lingjuan He, Hui Zhang, Xiuzhen Huang, Qiaozhen Liu, WenjuanPu, Libo Zhang, Yi Li, Huan Zhao, Zhifu Wang, Jianhong Zhu, Yu Nie, Shengshou Hu, David Sedmera, Zhong TP, Sean Wu, William Pu, Robert Anderson, Bin Zhou, Ying Yu, Li Zhang, Yan Yan, Zengyong Qiao, and Qing-Dong Wang. 2017. Identification of a hybrid myocardial zone in the mammalian heart after birth. Nature Communications. 8(1):87.
Zeng S., Zhong TP. 2017. Cardioascular Research in Zebrafish. Encyclopedia of Cardiovascular Research and Medicine. vol 4. p759-770. Vasan R, Sawyer D ed. Oxford: Elsevier,inc.
Peiyun Liu, Zhong TP. 2017. MAPK/ERK signalling is required for zebrafish cardiac regeneration. Biotechnol Lett.39(7):1069-77
Diqi Zhu, Yabo Fang, Kun Gao, Jie Shen, Zhong TP+ and Fen Li+. 2017. Vegfa Impacts Early Myocardium Development. Int J Mol Sci. 18(2). +Correspondent author
Da Wo, Jinhui Peng, Dan-ni Ren, Liman Qiu, Jinxiao Chen, Ye Zhu, Yingjing Yan,Hongwei Yan, Jian Wu, En Ma, Zhong TP, Yi-Han Chen, Zhong-Min Liu, Shangfeng Liu, Luoquan Ao, Zhenping Liu, Cizhong Jiang, Jun Peng, Yunzeng Zou, Qirong Qian, Weidong Zhu. 2016. Opposing Roles of Wnt Inhibitors IGFBP-4 and Dkk1 in Cardiac Ischemia by Differential Targeting of LRP5/6 and β–catenin. Circulation. 134(24):1991-2007.
Sun Jianjian, Zhihan Wu and Zhong TP. 2016. Cila Function in Cell Signaling and Organ Development. Scientia Sinica Vitae. 46,4:354-62
Peng XW, He QZ, Li GB, Ma JM, Zhong TP. 2016. Rac1-PAK2 pathway is essential for heart regeneration. Biochem Biophys Res Commun. 472:637-642.
Li Q, Yang H, Zhong TP. 2015. Regeneration across Metazoan Phylogeny: Lessons from Model Organisms. J Genet Genomics. 42:57-70.
Nan Wu, Xuan Ming, Jianqiu Xiao, Zhihong Wu, Xiaoli Chen, Marwan Shinawi, Yiping Shen, Guangju Yu, Jiaqi Liu, Hua Xie, Zoran S. Gucev, Sen Liu, Nan Yang, Hussam Al-Kateb, Jun Chen, Jian Zhang, Natalie Hauser, Ting Zhang, Velibor Tasic, Pengfei Liu, Xinlin Su, Xuedong Pan, Chunyu Liu, Liwen Wang, Joseph Shen, Jianxiong Shen, Yulin Chen, Ting Zhang, Jianguo Zhang, Kwong Wai Choy, Jun Wang, Qiqi Wang, Shugang Li, Weichen Zhou, Jin Guo, Yipeng Wang, Cheng Zhang, Hong Zhao, Yu An, Yu Zhao, Jiucun Wang, Zhenlei Liu, Yuzhi Zuo, V. Reid Sutton, Hongyan Wang, Yue Ming, Shashikant Kulkarni, Zhong TP, Sau Wai Cheung,Xue Zhang, Li Jin, James R. Lupski, Guixing Qiu, and Feng Zhang. 2015. Compound Inheritance of TBX6 Rare Null Variants and a Common Hypomorphic Allele in Congenital Scoliosis. New England Journal of Medicine. 372(4):341-50.
Jin D, Liu PY, Zhong TP. 2015. Prostaglandin Signaling in Ciliogenesis during Development. Cell Cycle. 14 (1):1-2.
Tian X, Hu T, Zhang H, He L, Huang X, Liu Q, Yu W, He L, Yang Z, Yan Y, YangX, Zhong TP, Pu WT, Zhou B. 2014. De Novo formation of a distinct coronary vascular population in neonatal heart. Science. 345 (6192): 90-4.
Du X, Shi H, Li J, Dong Y, Liang J, Ye J, Kong S, Zhang S, Zhong TP, Yuan Z, Xu T, Zhuang Y, Zheng B, Geng JG, Tao W. 2014. Mst1/Mst2 regulate development and function of regulatory T cells through modulation of Foxo1/Foxo3 stability in autoimmune disease. J Immunol. 192(4):1525-35.
Jin D, Ni TT, Sun J, Wan H, Amack JD, Yu G, Fleming J, Chiang C, Li W, Papierniak A, Cheepala S, Conseil G, Cole SP, Zhou B, Drummond IA, Schuetz JD, Malicki J, Zhong TP. 2014. Prostaglandin signaling regulates ciliogenesis and heart development by modulating intraflagellar transport. Nature Cell Biology.16 (9):841-51. (Article & Cover story; Previews by Barbry P & Zaragosi L E : An ABC of ciliogenesis. 2014. Nature Cell Biololgy. 16(9):826-7).
Dai X, She P, Chi F, Feng Y, Liu H, Jin D, Zhao Y, Guo X, Jiang D, Guan KL, Zhong TP, Zhao B. 2013. Phosphorylation of angiomotin by Lats1/2 kinases inhibits F-actin binding, cell migration and angiogenesis. J Biol Chem. 288 (47):34041-51.
Sun YP, Dong ZQ, Jin TH, Ang KK, Huang M, Haston KM, Peng J, Zhong TP, Finkbeiner, S, Weiss WA, Jan LY, Guo S. 2013. Imaging-based chemical screening reveals activity-dependent neural differentiation of pluripotent stem cells. Elife. e00508 (HHMI Press)
Cheepala SB, Bao J, Nachagari D, Sun D, Wang Y, Zhong TP, Naren AP, Zheng J, Schuetz JD. 2013. Crucial role for phylogenetically conserved cytoplasmic loop3 inABCC4 expression. J Biol Chem. 288(31):22207-18.
Tian X, Hu T, Zhang H, He L, Huang X, Liu Q, Yu W, He L, Yang Z, Zhang Z, Zhong TP, Yang X, Yang Z, Yan Y, Baldini A, Sun Y, Lu J, Schwartz RJ, Evans SM, Gittenberger-de Groot AC, Red-Horse K, Zhou B. 2013. Subepicardial endothelial cells invade the embryonic ventricle wall to form coronary arteries. Cell Research. 14 (5):371-45 (NaturePress)
Xiang Y, Cho PH, Zeng X, Zheng XJ, Jessen JR, Zhong TP, Xu XL. 2013. Trapping Cardiac Recessive Mutants via Expression-based Insertional Mutagenesis Screening. Circulation Research. 112 (4):606-17
Jin DQ, Li Q and Zhong TP. 2013. Chemical Genetics in Cardiomyocyte Generation. Chapter III in “Chemical Biology in Regenerative Medicine: Bridging Stem Cells and Future Therapies”. Wiley Publication, inc. USA
Ni TT, Lu J, Zhu M, Maddison LA, Boyd KL, Huskey L, Ju B, Hesselson D, Zhong TP, Page- McCaw PS, Stainier DY, Chen W. 2012. Conditional control of gene function by an invertible gene trap in zebrafish. Proc Natl Acad Sci. 109:15389-94.
Ni TT, Rellinger E, Williams C, Stephens L, Hu JY, Kim K, Marnett L, Heaton W, Hatzopoulos A, Zhong TP.2011. Discovering small molecules that promote cardiomyocyte generation via modulating Wnt signaling. Chemistry & Biology. 8:1658-68 (CellPress). (Previews by Ruey J. Yeh: A Wnt Inhibitors with a Twist. Chemistry & Biology. 18:1518-1520, 2011; Lab Reports, JAMA 307:5, 446, 2012)
Chopra SS, Watanabe V, Yang T, Stroud DM, Burns CG, Wells S, Zhong TP+, Roden DM. 2010. A non-electrogenic requirement for voltage-gated sodium channels in zebrafish heart development. Circulation Research. 106:1342-50. +Correspondent author
Williams C, Kim SH, Ni TT, Mitchell L, Ro HJ, Penn J, Baldwin HS, Solnica-Krezel L, Zhong TP.2010. Hedgehog signaling induces arterial endothelial cell formation by repressing venous cell fate. Developmental Biology. 341,196-204
Jin D, Ni TT, Hou J, Rellinger E, Zhong TP. 2009. Promoter analysis of ventricular myosin heavy chain (vmhc) in zebrafish embryos. Developmental Dynamics. 238,1760-1767
Zeng X, Zheng XJ, Xiang Y, Cho PH, Jessen JR, Zhong TP, Solnica-Krezel L and Brown AL. 2009. Phospholipase D1 is required for angiogenesis of intersegmental blood vessels in zebrafish. Developmental Biology. 328,363-76
Ni TT, William L, Shyr Y, Zhong TP. 2008. Use of normalization methods for analysis of microarrays containing ahigh degree of gene effects. BMC Bioinformatics. 9, 505.
Qu XH, Jia HB, Garrity DM, Tompkins K, Batts L, Appel B, Zhong TP+, Baldwin S. 2008. Ndrg4is required for normal myocyte proliferation during early cardiac development in zebrafish. Developmental Biology. 317, 486-96. +Correspondent author
Wang YX, Qian LX, Liu D, Yao LL, Gui YH, Zhong TP. Song HY. 2007. Bone morphogenetic protein-2 acts upstream of myocyte-specific enhancer factor 2a to control embryonic cardiac contractility. Cardiovascular Research. 74 (2):290-303.
Chopra S, Hiroshi Watanable, Zhong TP+, Roden D. 2007. Molecular cloning and analysis of zebrafish voltage-gated sodium channel b-subunits: Implication for the evolution of electrical signaling in vertebrates. BMC Evolution Biology. 7, 113-7. +Correspondent author.
Chopra S and Zhong TP. 2007. Vascular Development in Zebrafish. Endothelial Biomedicine. 1107-1127. Cambridge: Cambridge University Press, UK.
Jia H, King I, Chopra S, Wan H, Ni T, Jiang C, Guan X, Well S, Srivastava D and Zhong TP. 2007. Vertebrate heart growth is regulated by functional antagonism between Gridlock and Gata5. Proc Natl Acad Sci. 104,14008-14013.
Campbell WA, Yang H, Zetterberg H, Baulac S, Sears JA, Liu T, Wong STC, Zhong TP, XiaW. 2006. Zebrafish lacking Alzheimer presenilin enhancer 2 (Pen-2) demonstrate excessive p53 dependent apoptosis and neuronal loss. J Neurochemistry. 96,1423-40.
Rutenberg JB, Fischer A., Jia HB, Gessler M., Zhong TP and Mercola M. 2006. Developmental patterning of the cardiac atrioventricular canal by Notch and Hairy-related transcription factors. Development. 133,4381-90.
Wang Y, Zhong TP, Qian L, Dong Y, Jiang Q, Tan L, Song H. 2005. Wortmannin induces zebrafish cardia bifida through a mechanism independent of phosphoinositide 3-kinase and myocisn light chain kinase. Biochem Biophys Res Commun. 331 (1):303-8
Zhang L, Zhong TP, Wang Y, Jiang Q, Song H, Gui Y. 2005. TBX1, a DiGeorge syndrome candidate gene, is inhibited by retinoic acid. Int. J. Developmental Biology. 50:55-61
Wang Y, Qian L, Zhang Y, Jiang Q, Dong Y, Liu X, Yang X, Zhong TP+, Song H. 2005. Requirements of myocyte-specific enhancer factor 2A in zebrafish cardiac contractility. FEBS. 579, 4843-50. +Correspondent author
Zhong TP. 2005. Zebrafish Genetics and Formation of Embryonic Vasculature. Current Topics in Development Biology. 71,53-81.
Peterson R, Shaw SY, Peterson TA, Milan DJ, Zhong Tao P, Schreiber S, Mac Rae C and Fishman, MC. 2004. Chemical suppression of a genetic mutation in a zebrafish model of aortic coarctation. Nature Biotechnology. 22, 593-599.
Xu X, Miller S, Zhong TP, Mohideen M, Crossley DA, Burggren WW and Fishman MC. 2002. Cardiomyopathy in zebrafish due to mutation in an alternatively spliced exon of Titin. Nature Genetics. 30, 205-209.
Zhong TP, Childes S, Leu J, Fishman MC. 2001. The Gridlock signaling pathway fashions the first embryonic artery. Nature. 414, 216-220. (Comment by George D. Yancopoulos: Gridlock in the blood. Nature. 404, 163-164)
Zhong TP, Rosenberg M, Mohideen M, Weinstein B, Fishman MC. 2000. Gridlock, an HLH gene required for assembly of the aorta in zebrafish. Science. 287, 1820-1824.
Amemiya C, Zhong TP, Silverman G, Fishman MC, Zon L. 1999. In the zebrafish: Genetics and Genomics. Methods in Cell Biol. H.W. Detrich, III, M. Westerfield, L.I. Zon, Eds. (Academic Press, San Diego) pp.236.
Zhong TP, Kaphingst K, Akella U, Haldi M, Lander E and Fishman MC. 1998. Zebrafish genomic library in yeast artificial chromosome. Genomics. 48, 136-138.
Zhong TP, Luke M and Arndt K. 1996. Transcriptional regulation of the yeast DnaJ molecule SIS1 gene. J. Biological Chemistry. 271, 1349-1356.
Zhong TP and Arndt K. 1993. Yeast SIS1 protein, a DnaJ homologue, is required for the Initiation of translation. Cell. 73, 1175-1186.
Sarabia M, Sutton A, Zhong TP and Arndt K. 1992. SIT4 protein phosphatase is required for the normal accumulation of SWI4, CLN1, CLN2, and HCS26 RNAs during Late G1. Genes & Development. 6, 2417-2428.
荣誉及奖励荣誉与业绩及专业学会会员: 国家杰出青年基金获得者 科学中国人年度人物 (2014) 国家重大科学研究计划心脏发育首席科学家 复旦大学医学院施李月卿杰出毕业生
国家基金会评审委员会专家 科技部中期评审委员会专家 美国国立卫生研究院(NIH)项目评审委员会专家 美国心脏学会(AHA)项目评审委员会专家 美国遗传学学会会员, 发育生物学学会会员, 心脏协会会员 |

|
钟涛 |