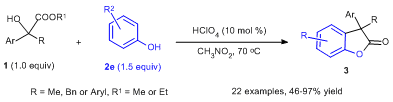About
EducationWorkExperienceResumeOther Appointments
(2012.8- ) Member of Advisory Board of of Organic & Biomolecular Chemistry (IF = 3.69) (2014.2- ) Member of Advisory Board of Organic Chemistry: Current Research (2014.5- ) Member of Advisory Board of Current Organocatalysis (2015.8- ) Member of Advisory Board of Acta Chim. Sinica, Research FieldsOur group focused on the development of new chiral catalysts and new catalytic asymmetric reactions allowing the catalytic economical and enantioselective construction of tetrasubstituted stereogenic carbon center. Currently‚ we are interested in the catalytic asymmetric economical synthesis of 3‚3-disubstittuted oxindoles, quaternary amino acids and compounds with a fluoroalkyl group substituted tetrasubstituted stereogenic carbon center. We also aim at the development of novel tandem reactions that internally recyle the by-products from the previous step to catalyze the downstream reaction‚ or to improve the reactivity and selectivity of the following transformation.
Prof. Dr. Jian Zhou Education
1993-1997 B.S. Sichuan Normal University 1999-2004 Ph.D. Shanghai Institute of Organic Chemistry‚ Chinese Academy of Science (Advisor, Professor Yong Tang) Academic Careers
2004.9-2005.8 Postdoctoral fellow‚ the Graduate School of Pharmaceutical Sciences‚ the University of Tokyo‚ Japan (Advisor: Professor Shu Kobayashi) 2005.10-2008.9 Postdoctoral fellow‚ Max-Planck-Institut für Kohlenforschung‚ Germany (Advisor: Professor Benjamin List) 2008.11-present Professor‚ Shanghai Key Laboratory of Green Chemistry and Chemical Processes‚ Department of Chemistry‚ East China Normal University Awards
2015, The CSJ Asian International Symposium Distinguished Lectureship Award Awarded by Chemistry Society of Japan (CSJ) 2014, Fellow of Royal Society of Chemistry) 2011 “Thieme Chemistry Journals Award”
Enrollment and TrainingCourseScientific ResearchAcademic AchievementsPublications since independent work(from November 2008):
121) Z. Zhang, Y. Sun, Y. Gong, D.-L. Tang, H. Luo, Z.-P. Zhao, F. Zhou*, X. Wang* & J. Zhou* Enantioselective propargylic amination and related tandem sequences to α-tertiary ethynylamines and azacycles Nat. Chem. 2024, 10.1038/s41557-024-01479-z. 120) P.-W Xu, F. Zhou*, L. Zhu* & J. Zhou* Catalytic desymmetrization reactions to synthesize all-carbon quaternary stereocentresNature Synthesis2023, 2, 1020-1036. 119) Y. Gao, X.-X. Zhang, J.-S. Yu, J. Zhou* Recent Advances in Catalytic Enantioselective Synthesis of α -Chiral Tertiary AzidesActa Chim. Sinica 2023, 81, 1590-1608. 118) J.-S. Tian, Z. Tu, F. Zhou, J.-S. Yu and J. Zhou* Tandem imine generation/N-cyclization/C-alkylation sequence to access N-functionalized indoles featuring an aza-quaternary carbon Org. Chem. Front.2023, 10, 1759. 117) Y. Gong, C. Wang, F. Zhou,* K. Liao, X.-Y. Wang, Y. Sun, Y.-X. Zhang, Z. Tu, X. Wang,* and J. Zhou* Sulfonyl-PYBOX Ligands Enable Kinetic Resolution of α-Tertiary Azides by CuAACAngew. Chem. Int. Ed. 2023, e202301470. 116) C.-W. Lei, X.-Y. Wang, B.-S. Mu, J.-S. Yu,* Y. Zhou,* and J. Zhou* Me2(CH2Cl)SiCF3 Facilitated Tandem Synthesis of Oxasilacycles Featuring a Trifluoromethyl GroupOrg. Lett. 2022, 24, 8364−8369. 115) B.-W. Pan, Y. Shi, S.-Z. Dong, J.-X. He, B.-S. Mu, W.-B. Wu, Y. Zhou,* F. Zhou * and J. Zhou* Highly stereoselective synthesis ofspirocyclopropylthiooxindoles and biological evaluationOrg. Chem. Front.2022, 9, 2640. 114) X.-Y Cui, F. Zhou,* H.-H. Wu,* J. Zhou* Asymmetric Tandem Reactions Achieved by Chiral Amine & Gold(I) Cooperative Catalysis, Chin. J. Org. Chem. 2022, 42, 3033-3050. 113) W.-B. Wu,X. Yu, J.-S. Yu, X. Wang*, W.-G. Wang* & J. Zhou* Constructing Tertiary Alcohols with Vicinal Stereocenters: Highly Diastereo- and Enantioselective Cyanosilylation of α-Branched Acyclic Ketones and Their Kinetic Resolution, CCS Chem. 2022, 4, 2140–2152. 112) B.-S. Mu, Y. Gao, F.-M. Yang, W.-B. Wu, Y. Zhang, X. Wang,* J.-S. Yu,* and J. Zhou* The Bifunctional Silyl Reagent Me2(CH2Cl)SiCF3 Enables Highly Enantioselective Ketone Trifluoromethylation and Related Tandem ProcessesAngew. Chem. Int. Ed. 2022, e202208861. 111) B.-S. Mu, Z.-H. Zhang, W.-B. Wu*, J.-S. Yu, J. Zhou* Recent Advances in Synthesis of Chiral 1,2-DihydropyridinesActa Chim. Sinica 2021, 79, 685-693. 110) P.-W. Xu, X.-Y. Cui, C. Chen, F. Zhou, J.-S. Yu, Y.-F. Ao,* and J. Zhou* Enantioselective Synthesis of Cα-Tetrasubstituted N-Hydroxyl-α-amino Nitriles via Cyanation of Ketonitrones Using Me2(CH2Cl)SiCN, Org. Lett. 2021, 23, 8471−8476. 109) K Liao, Y. Gong, R.-Y. Zhu, C Wang, F. Zhou,* and J. Zhou* Highly Enantioselective CuAAC of Functional Tertiary Alcohols Featuring an Ethynyl Group and Their Kinetic ResolutionAngew. Chem. Int. Ed.2021, 60, 8488 –8493. 108) X.-Y. Cui, Y.-L. Zhao, Y.-M. Chen, S.-Z. Dong,* F. Zhou, H.-H. Wu,* and J. Zhou* Au-Catalyzed Formal Allylation of Diazo(thio)oxindoles: Application to Tandem Asymmetric Synthesis of Quaternary Stereocenters, Org. Lett. 2021, 23, 4864-4869. 107) B.-S. Mu, X.-Y. Cui, X.-P. Zeng, J.-S. Yu and J. Zhou*Modular synthesis of chiral 1,2-dihydropyridines via Mannich/Wittig/cycloisomerization sequence that internally reuses wasteNature Commun.2021, 12, 2219. 106) X.-S. Hu, J.-X. He, S.-Z. Dong, Q.-H. Zhao, J.-S. Yu and J. Zhou*Regioselective Markovnikov hydrodifluoroalkylation of alkenes using difluoroenoxysilanes. Nature Commun. 2020, 11, 5500. 105) P.-G. Ding, X.-S. Hu, J.-S. Yu* and J. Zhou*Diastereodivergent Synthesis of α-Chiral Tertiary Azides through Catalytic Asymmetric Michael Addition.Org. Lett. 2020, 22, 8578-8583. 104) Y. Shi, B.‐W. Pan, J.‐S. Yu. Y. Zhou, J. Zhou*Recent Advances in Applying Carbonyl‐stabilized Phosphorus Ylides for Catalysis.ChemCatChem, 2021, 13, 129-139. 103) F. Zhou*, L. Zhu, B.-W. Pan, Y. Shi, Y.-L. Liu, J. Zhou*Catalytic enantioselective construction of vicinal quaternary carbon stereocenters. Chem. Sci. 2020, 11, 9341–9365. 102) W.-B. Wu, J.-S. Yu, J. Zhou*Catalytic Enantioselective Cyanation: Recent Advances and PerspectivesACS Catal. 2020, 10, 7668−7690. 101) Q. Xiao, J.-S. Yu, Y.-F. Wang, D.-J. Ma, J. Zhou* and X. Lou* 3-Difluoroalkyl Quaternary Oxindoles Inhibit Macrophage Pyroptosis by Blocking Inflammasome Recruitment of Caspase-1.ACS Med. Chem. Lett. 2020, 11, 1392−1401. 100) C. Wang, F. Zhou, J. Zhou*Recent Advances in the Enantioselective Copper(I)-Catalyzed Azide-Alkyne Cycloaddition Reaction.Chin. J. Org. Chem. 2020, 40, 3065-3077. 99) F. Zhou*, J. Zhou*.Ni-Catalyzed Desymmetrization of Malononitriles to Cycloenones with a Nitrile-Containing All-Carbon Quaternary Center.Chin. J. Org. Chem. 2020, 40, 2180-2181. 98) P.-G. Ding, F. Zhou, X. Wang, Q.-H. Zhao, J.-S. Yu and J. Zhou*. H-Bond donor-directed switch of diastereoselectivity in the Michael addition of α-azido ketones to nitroolefins.Chem. Sci. 2020, 11, 3852-3861. 97) C. Chen, W.-B. Wu, Y.-H. Li, Q.-H. Zhao, J.-S. Yu, J. Zhou*Activation of Chiral (Salen)TiCl2 Complex by Phosphorane for the Highly Enantioselective Cyanation of Nitroolefins.Org. Lett. 2020, 22, 2099−2104. 96) W.-B. Wu, X.-P. Zeng, J. Zhou*Carbonyl-Stabilized Phosphorus Ylide as an Organocatalyst for Cyanosilylation Reactions Using TMSCN.J. Org. Chem. 2020, 85, 14342-14350. 95) R.-Y. Zhu, K. Liao, J.-S. Yu and J. Zhou* Recent Advances in Catalytic Asymmetric Synthesis of P-Chiral Phosphine Oxides Acta Chim. Sinica 2020, 78, 193-216. 94) C. Wang, R.-Y. Zhu, K. Liao, F. Zhou, J. Zhou* Enantioselective Cu(I)-Catalyzed Cycloaddition of Prochiral Diazides with Terminal or 1-Iodoalkynes.Org. Lett. 2020‚ 22, 1270-1274. 93) Y.-H. Wang, Z.-Y. Cao, Q.-H. Li, G.-Q. Lin, J. Zhou* and P. Tian* Activating Pronucleophiles with High pKa Values: Chiral Organo-Superbases. Angew. Chem. Int. Ed. 2020, 59, 8004-8014. 92) Y.-H. Wang, J.-S. Tian, P.-W. Tan, Q. Cao, X.-X. Zhang, Z.-Y. Cao, F. Zhou, X. Wang, and J. Zhou* Regiodivergent Intramolecular Nucleophilic Addition of Ketimines for the Diverse Synthesis of Azacycles.Angew. Chem. Int. Ed. 2020, 59, 1634 –1643. 91)R.-Y. Zhu, L. Chen, X.-S. Hu, F. Zhou and J. Zhou* Enantioselective synthesis of P-chiral tertiaryphosphine oxides with an ethynyl group via Cu(I)-catalyzed azide–alkyne cycloaddition.Chem. Sci. 2020, 11, 97–106. 90) S.-L. Xie, X.-T Gao, F. Zhou, H.-H. Wu, J. Zhou* Enantioselective carboxylative cyclization of propargylic alcohol with carbondioxide under mild conditions. Chin. Chem. Lett. 2020, 31, 324–328. 89) Y. Gong, Z.-Y. Cao, Y.-B. Shi, F. Zhou, Y. Zhou and J. Zhou* A highly efficient Hg(OTf)2-mediated Sakurai–Hosomiallylation of N-tert butyloxycarbonylamino sulfones, aldehydes, fluoroalkylketones and α,β-unsaturated enones usingallyltrimethylsilane. Org. Chem. Front. 2019, 6, 3989–3995. 88) Y.-P. Tian, Y. Gong, X.-S. Hu, J.-S. Yu, Y. Zhou and J. Zhou*HClO4 catalysed aldol-type reaction of fluorinated silyl enol ethers with acetals or ketalstoward fluoroalkyl ethers.Org. Biomol. Chem. 2019, 17, 9430–9434. 87) X.-S. Hu, J.-S. Yu and J. Zhou* Catalytic selective mono- and difluoroalkylation using fluorinated silyl enol ethers.Chem.Commun. 2019, 55, 13638-13648. 86) X.-T. Gao, S.-L. Xie, F. Zhou, H.-H. Wu and J. Zhou* Multifunctional 1,3-diphenylguanidine for the carboxylativecyclization of homopropargyl amines with CO2 under ambient temperature and pressure.Chem. Commun. 2019, 55, 14303-14306. 85) S.-L. Xie, X.-Y. Cui, X.-T. Gao, F. Zhou, H.-H. Wu and J. Zhou* Stereoselective defluorinative carboxylation of gem-difluoroalkenes with carbon dioxide.Org. Chem. Front. 2019, 6, 3678-3682. 84) P.-W. Xu, J.-S. Yu,* C. Chen, Z.-Y. Cao, F. Zhou and J. Zhou*. Catalytic Enantioselective Construction of Spiro Quaternary Carbon Stereocenters.ACS Catalysis 2019, 9, 1820-1882. 83) K. Liao, X.-S. Hu, R.-Y. Zhu, R.-H. Rao, J.-S. Yu, F. Zhou and J. Zhou* Catalytic Enantioselective Protonation of Monofluorinated Silyl Enol Ethers towards Chiral α-Fluoroketones. Chin. J. Chem. 2019, 37, 799-806. 82) Y.-J.Hao, Y. Gong, Z.-Y Cao, Y. Zhou, J. Zhou* A highly efficient In(OTf)3-catalyzed[3+3] annulation of spirocyclopropyl oxindoles with 1,4-di-thiane-2,5-diol.Chin. Chem. Lett.2020, 31, 681-684. 81) X.-S Hu, P.-G. Ding, J.-S. Yu and J. Zhou* A Sc(OTf)3 catalyzed Mukaiyama–Mannich reaction of difluoroenoxysilanes with unactivatedketimines. Org. Chem. Front. 2019, 6, 2500-2505. 80) Y. Gong, J.-S. Yu, Y.-J. Hao, Y. Zhou, J. Zhou* Catalytic Enantioselective Aldol-Type Reaction Using α-Fluorinated Enolates. Asian J. Org. Chem. 2019, 8, 610–626. 79) X.-S. Hu, J.-S. Yu,* Y. Gong, J. Zhou*Internally reuse by-product as promoter: A catalyst-free imine formation/Mukaiyama-Mannich sequence of α-amido sulfones with fluorinated silyl enol ethers. J. Fluorine Chem. 2019, 219, 106-114. 78) Y.-J. Hao, J.-S. Yu, Y. Zhou, W. Wang, J. Zhou*C―F…H―X相互作用在有机反应中的影响(Influence of C—F…H—X Interactions on Organic Reactions)Acta Chim. Sinica 2018, 76, 925—939 77) Y.-J. Hao, X.-S. Hu, J.-S. Yu, Y. Zhou*, F. Zhou, J. Zhou*An efficient Fe(III)-catalyzed 1,6-conjugate addition of para-quinone methides with fluorinated silyl enol ethers toward β,β-diaryl α-fluorinated ketones.Tetrahedron 2018, 74, 7395-7398. 76) F.-M. Liao, Y. Du, F. Zhou, J. Zhou* Au(I)/手性双功能叔胺催化实现的一锅法串联成烯/不对称环化反应构建螺环季碳氧化吲哚(Au(I)/Chiral Tertiary Amine Catalyzed Tandem Olefination/Asymmetric Cyclization Reaction to Quaternary Spirocyclic Oxindoles) Acta Chim. Sinica 2018, 76, 862—868. 75) Z.-Y. Cao, W. Wang, K. Liao, X. Wang, J. Zhou* and J. Ma* Catalytic enantioselective synthesis of cyclopropanes featuring vicinal all-carbon quaternary stereocenters with a CH2F group; study of the influence of C–F⋯H–N interactions on reactivityOrg. Chem. Front. 2018, 5, 2960-2968. 74) P.-W. Xu, C. Chen, J.-K. Liu, Y.-T. Song, F. Zhou, J. Yan and J. Zhou* One-Pot Sequential [3 + 3] Dipolar Cycloaddition of Aldehyde or Ketone and Hydroxylamine with Spirocyclopropyl Oxindole.J. Org. Chem. 2018, 83, 12763-12774 73) Z.-Y. Cao, F. Zhou and J. Zhou* Development of Synthetic Methodologies via Catalytic Enantioselective Synthesis of 3,3-Disubstituted OxindolesAcc. Chem. Res. 2018, 51, 1443-1454. 72) P.-G. Ding, X.-S. Hu, F. Zhou and J. Zhou*Catalytic enantioselective synthesis of α-chiral azidesOrg. Chem. Front. 2018, 5, 1542-1559. 71) Y.-L. Liu, X.-P. Yin, J. Zhou* Internally Reuse Waste: Catalytic Asymmetric One-Pot Strecker Reaction of Fluoroalkyl Ketones, Anilines and TMSCN by Sequential CatalysisChin. J. Chem. 2018, 36, 321-328. 70) X.‐P. Yin, L. Zhu, J. Zhou* Metal‐Free Azidation of α‐Hydroxy Esters and α‐Hydroxy Ketones Using AzidotrimethylsilaneAdv. Synth. Catal. 2018, 360, 1116-1122. 69) X.-T. Gao, C.-C. Gan, S.-Y. Liu, F. Zhou*, H.-H. Wu,* J. Zhou* Utilization of CO2 as a C1 Building Block in a Tandem Asymmetric A3 Coupling-Carboxylative Cyclization Sequence to 2-OxazolidinonesACS Catal. 2017, 7, 8588-8593. 68) F.-M. Liao,X.-T. Gao, X.-S. Hu, S.-L. Xie, J. Zhou*A general and efficient Lewis acid catalysed Mukaiyama-aldol reaction of difluoroenoxysilanes and ketones. Sci. Bull. 2017, 62, 1504-1509. 67) P.-W. Xu, J.-K. Liu,L. Shen, Z.-Y. Cao, X.-L. Zhao, J. Yan,* J. Zhou*Diastereo- and enantioselective [3 + 3] cycloaddition of spirocyclopropyl oxindoles using both aldonitrones and ketonitrones. Nature Communications, 2017, 8, 1619. 66) F.Zhou,* S.-L.Xie, X.-T.Gao, R. Zhang,* C.-H.Wang, G.-Q.Yin, J. Zhou* Activation of (salen)CoI complex by phosphorane for carbon dioxide transformation at ambient temperature and pressure. Green Chem. 2017, 19, 3908-3915. 65) F.-M. Liao, J.-S. Yu and J. Zhou* Recent Advances in the Highly Stereoselective Synthesis of Tri- or Tetra-substituted MonofluoroalkenesChin. J. Org. Chem.2017, 37, 2175-2186. 64) X.-S. Hu, Y. Du, J.-S. Yu,* F.-M. Liao, P.-G. Ding and J. Zhou* A Highly Efficient Gold(I)-Catalyzed Mukaiyama–Mannich Reaction of α-Amino Sulfones with Fluorinated Silyl Enol Ethers To Give β-Amino α-Fluorinated KetonesSynlett 2017, 16, 2194-2198. 63) F.-M. Liao, Z-Y. Cao, J.-S. Yu and J. Zhou* Highly Stereoselective Gold-Catalyzed Coupling of Diazo Reagents and Fluorinated Enol Silyl Ethers to Tetrasubstituted AlkenesAngew. Chem. Int. Ed. 2017‚ 56,2459-2463. 62) X. Ye, X.-P. Zeng, J. Zhou, Me2(CH2Cl)SiCN和Me3SiCN在醛的不对称氰化反应中的比较研究, Acta Chim. Sinica 2016, 74, 984—989 61) Y.-H. Wang, Z.-Y. Cao, J. Zhou* Nucleophilic Difluoromethylenation of Ketones Using Diethyl Difluoro(trimethylsilyl)methylphosphonate Mediated by 18-Crown-6 Ether/KOAc J. Org. Chem.2016, DOI: 10.1021/acs.joc.6b01457 60) X.-P. Zeng, J. Zhou* Me2(CH2Cl)SiCN: Bifunctional Cyanating Reagent for the Synthesis of Tertiary Alcohols with a Chloromethyl Ketone Moiety via Ketone Cyanosilylation J. Am. Chem. Soc. 2016, 138, DOI:10.1021/jacs.6b05601. 59) J.-S. Yu, H.-M. Huang, P.-G. Ding, X.-S. Hu, F. Zhou, J. Zhou*Catalytic Enantioselective Construction of Sulfur-Containing Tetrasubstituted Carbon Stereocenters ACS Catal. 2016, 6, DOI:10.1021/acscatal.6b01496. 58) X.-P. Zeng, Z.-Y. Cao, Y.-H.Wang, F. Zhou, J. Zhou*Catalytic Enantioselective Desymmetrization Reactions to All-Carbon Quaternary Stereocenters Chem. Rev. 2016. 116, 7330. 57) Y.-L. Zhao, Z.-Y. Cao, X.-P. Zeng, J.-M. Shi, Y.-H. Yu and J. Zhou* Asymmetric sequential Au(I)/chiral tertiary amine catalysis: an enone-formation/cyanosilylation sequence to optically active 3-alkenyloxindoles from diazooxindoles Chem. Commun., 2016, 52, 3643-3646. 56) J.-S. Yu, J. Zhou*Organocatalytic enantioselective Mukaiyama–Mannich reaction of fluorinated enol silyl ethers and cyclicN-sulfonyl ketimines Org. Chem. Front. 2016, 3, 298-303. 55) Z.-Y. Cao, Y.-L. Zhao, J. Zhou*Sequential Au(I)/chiral tertiary amine catalysis: a tandem C–H functionalization of anisoles or a thiophene/asymmetric Michael addition sequence to quaternary oxindoles Chem. Commun. 2016, 52, 2537 54) Z.-Y. Cao, J.-S. Jiang, J. Zhou*A highly enantioselective Hg(II)-catalyzed Sakurai–Hosomi reaction of isatins with allyltrimethylsilanes Org. Biomol. Chem. 2016, 14, 5500-5504 (invited for New Talent Issue). 53) X.-P. Zeng,Z.-Y. Cao,X. Wang,L. Chen,F. Zhou,F. Zhu,C.-H. Wang, J. Zhou*Activation of Chiral (Salen)AlCl Complex by Phosphorane for Highly Enantioselective Cyanosilylation of Ketones and Enones J. Am. Chem. Soc. 2016, 138, 416-425. 52) J.-S. Yu, J. Zhou* A highly efficient Mukaiyama–Mannich reaction ofN-Boc isatin ketimines and other active cyclic ketimines using difluoroenol silyl ethers catalyzed by Ph3PAuOTf Org. Biomol. Chem. 2015, 13, 10968 (Feature article). 51) J.-S. Yu, F. Zhou, Y.-L. Liu, J. Zhou* A Journey in the Catalytic Synthesis of 3-Substituted 3-Aminooxindoles Synlett 2015, 26, 2491-2504. (invited account) 50) Z.-Y. Cao, J. Zhou* Catalytic asymmetric synthesis of polysubstituted spirocyclopropyl oxindoles: organocatalysis versus transition metal catalysis Org. Chem. Front.2015, 2, 849-858. 49) F.-M. Liao,Y.-L. Liu, J.-S. Yu, F. Zhou, J. Zhou* An efficient catalyst-free Mukaiyama-aldol reaction of fluorinated enol silyl ethers with tryptanthrin Org. Biomol. Chem., 2015,13, 8906-8911. 48) X.-P.Yin, P.-W. Xu, K. Dong, K. Liao, F. Zhou, J. Zhou* Ga(OTf)3催化的3-羟基氧化吲哚与TMSN3的取代反应研究 Acta Chim. Sinica,2015, 73, 685-689. 47) J.-S. Yu, F.-M. Liao, W.-M. Gao, K. Liao, R.-L. Zuo and J. Zhou* Michael Addition Catalyzed by Chiral Secondary Amine Phosphoramide Using Fluorinated Silyl Enol Ethers: Formation of Quaternary Carbon Stereocenters Angew. Chem. Int. Ed. 2015‚54, 7381. 46) L. Chen, Y. Du, X.-P. Zeng, T.-D. Shi, F. Zhou and J. Zhou* Successively Recycle Waste as Catalyst: A One-Pot Wittig/1,4-Reduction/Paal–Knorr Sequence for Modular Synthesis of Substituted Furans Org. Lett.2015‚ 17, 1557. 45) Zhu, P.-W. Xu, F. Zhou, C.-H. Wang* and J. Zhou* Recycle Waste Salt as Reagent: A One-Pot Substitution/Krapcho Reaction Sequence to α-Fluorinated Esters and Sulfones Org. Lett.2015‚ 17, 972. 44) Y.-L. Liu, J. Zhou*, Catalytic Asymmetric Strecker Reaction: Bifunctional Chiral Tertiary Amine/Hydrogen-Bond Donor Catalysis Joins the Field Synthesis, 2015, 1210-1226 (invited mini-review) 43) W.-M. Gao, J.-S. Yu, Y.-L. Zhao, Y.-L. Liu, F. Zhou, H.-H. Wu,* J. Zhou* Highly enantioselective Michael addition of 3-arylthio- and 3-alkylthiooxindoles to nitroolefins catalyzed by a simple cinchona alkaloid derived phosphoramide Chem. Commun.2014, 50, 15719. 42) X.-P. Yin, X.-P. Zeng, Y.-L. Liu, F.-M. Liao, J.-S. Yu, F. Zhou, J. Zhou* Asymmetric Triple Relay Catalysis: Enantioselective Synthesis of Spirocyclic Indolines through a One-Pot Process Featuring an Asymmetric6π Electrocyclization Angew. Chem. Int. Ed.2014‚53, 13740. 41) F. Zhou, F.-M. Liao, J.-S. Yu, J. Zhou* Catalytic Asymmetric Electrophilic Amination Reactions To Form Nitrogen-Bearing Tetrasubstituted Carbon Stereocenters Synthesis, 2014, DOI: 10.1055/s-0034-1379255 (Invited focus review). https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0034-1379255 40) J.-S. Yu, Y.-L. Liu, J. Tang, X. Wang,* J. Zhou* Highly Efficient “On Water” Catalyst-Free Nucleophilic Addition Reactions Using Difluoroenoxysilanes: Dramatic Fluorine Effects Angew. Chem. Int. Ed.2014‚53, 9512-9516. 39) Y.-L. Liu, F.-M. Liao, Y.-F. Niu, X.-L. Zhao, J. Zhou, Highly stereoselective construction of adjacent tetrasubstituted carbon stereogenic centres via organocatalytic Mukaiyama-aldol reaction of monofluorinated silyl enol ethers to isatins Org. Chem. Front.2014, 1, 742-747. 38) L. Chen, X.-P. Yin, C.-H. Wang, J. Zhou* Catalytic functionalization of tertiary alcohols to fully substituted carbon centresOrg. Biomol. Chem.2014‚ 12, 6033-6048(Invited review) 37) Y.-H. Wang, Z.-Y. Cao,Y.-F. Niu, X.-L. Zhao,J. Zhou* 高对映选择性的有机催化的硝基烷烃对N-Boc靛红亚胺的不对称aza-Henry反应 Acta Chim. Sinica.,2014, 72, 867-872. (Invited for special issue on organocatalysis) 36)Z.-Y. Cao, Y.-H. Wang, X.-P. Zeng,J. Zhou*, Catalytic asymmetric synthesis of 3,3-disubstituted oxindoles: diazooxindole joins the field Tetrahedron Lett. 2014, 55, 2571-2584(Invited Digest Paper). 35) Y.-H. Wang, Y.-L. Liu, Z.-Y. Cao,J. Zhou*, An Organocatalytic Addition of Nitromethane to Activated Ketimines Asian J. Org. Chem.2014, 3, 429-432. (Invited for special issue on organocatalysis) 34) Y.-L. Liu, X. Wang, Y.-L. Zhao, F. Zhu, X.-P. Zeng,L. Chen, C.-H. Wang,X.-L. Zhao, J. Zhou* One-Pot Tandem Approach to Spirocyclic Oxindoles Featuring Adjacent Spiro-Stereocenters. Angew. Chem. Int. Ed.2013‚ 52‚ 13735-13739. (Highlighted by Synfacts2014‚ 10, 204) 33) L. Chen, F. Zhu, H.-C. Wang,* J. Zhou*, A highly efficient thiourea catalyzed dehydrative nucleophilic substitution reaction of 3-substituted oxindoles with xanthydrols RSC Adv. 2013, 3, 19880-19884. 32) F. Zhou, C. Tan, J. Tang, Y.-Y. Zhang, W.-M. Gao, H.-H. Wu, Y.-H. Yu and Jian Zhou,* Asymmetric Copper(I)-Catalyzed Azide–Alkyne Cycloaddition to Quaternary OxindolesJ. Am. Chem. Soc. 2013‚ 135‚DOI: 10.1021/ja4066656 31)Y.-L. Liu, F. Zhu, C.-H. Wang and Jian Zhou,* Asymmetric synthesis of 3-hydroxyoxindoles:Transition metal catalysis vs Organocatalysis Chin. J. Org. Chem.2013,in press (invited focus review)。 30) Z.-Y. Cao, X. Wang, C. Tan, X.-L. Zhao, J. Zhou,* and K. Ding Highly Stereoselective Olefin Cyclopropanation of Diazooxindoles Catalyzed by a C2-Symmetric Spiroketal Bisphosphine/Au(I) Complex J. Am. Chem. Soc. 2013‚ 135‚ DOI: 10.1021/ja4040895. 29) C.-B. Ji, Z.-Y. Cao, X. Wang,* D.-Y. Wu and Jian Zhou,* A Catalyst-Free, One-Pot Three-Component Aminomethylation of α-Substituted Nitroacetates: Theoretical and Experimental Studies into the Rate-Accelerating Effects of the Solvent Methanol. Chem. Asian. J.2013, 8, 877. 28) F. Zhou, X.-P. Zeng, C. Wang, X.-L. Zhao and Jian Zhou,* Organocatalytic asymmetric synthesis of 3,3-disubstituted oxindoles featuring two heteroatoms at C3 position Chem. Commun.2013, 49, 2022-2024. 27) Y.-L. Liu, J.-S. Yu, Jian Zhou,* Catalytic Asymmetric Construction of Stereogenic Carbon Centers that Feature a gem-Difluoroalkyl Group Asian J. Org. Chem. 2013, 2, 194-206. (Invited focus review) 26) L. Chen, T.-D. Shi, Jian Zhou,* Waste as Catalyst: Tandem Wittig/Conjugate Reduction Sequence to α-CF3 γ-Keto Esters That Uses Ph3PO as Catalyst for the Chemoselective Conjugate ReductionChem. Asian. J.2013, 8, 556-559. 25) Z.-Y. Cao, F. Zhou, Y.-H. Yu, J. Zhou*, A Highly Diastereo- and Enantioselective Hg(II)-Catalyzed Cyclopropanation of Diazooxindoles and AlkenesOrg. Lett.2013‚ 15, 42. (Highlited by Synfacts2013‚ 9‚ 415). 24) X.-P. Zeng, Y.-L. Liu, C.-B. Ji, J. Zhou*, The First Catalytic Asymmetric Morita-Baylis-Hillman Reaction of Acrolein with Aromatic Aldehydes , Chin. J. Chem. 2012, 30, 2631-2635 (Feature article) 23) Y.-L. Liu, J. Zhou*, Organocatalytic asymmetric cyanation of isatin derived N-Boc ketoimines Chem. Commun.2013‚ 49, 4421. (Invited for themed issue for 2013 Emerging Investigator). (Highlited by Synfacts2013‚ 9‚ 96). 22) L. Chen, J. Zhou* A Highly Efficient Friedel–Crafts Reaction of Tertiary α-Hydroxyesters or α-Hydroxyketones to α-Quaternary Esters or Ketones. Chem. Asian J.2012‚ 7‚ 2510-2515. (VIP paper). 21) F. Zhu, F. Zhou, Z.-Y. Cao, C. Wang, Y.-X. Zhang, C.-H. Wang*, J. Zhou*A Facile Method for the Synthesis of 3-Substituted 3-(Alkylthio)oxindoles or 3-Alkoxyoxindoles Synthesis, 2012, 3129-3144. (invited feature article). 20) J.-S. Yu, F. Zhou, Y.-L. Liu, J. Zhou* Organocatalytic asymmetric Michael addition of unprotected 3-substituted oxindoles to 1,4-naphthoquinone.Beilstein. J. Org. Chem.2012, 8, 1360-1365. (invited paper for a Thematic Series entitled “Organocatalysis”) 19) Y.-L. Liu‚ X.-P. Zeng‚ J. Zhou*高对映选择性的有机催化的二氟烯醇硅醚与β‚γ-不饱和-α-酮酸酯的不对称Mukaiyama-aldol 反应 《化学学报》‚2012‚ 70‚ 1451-1456。
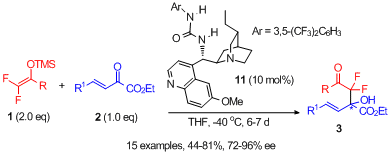
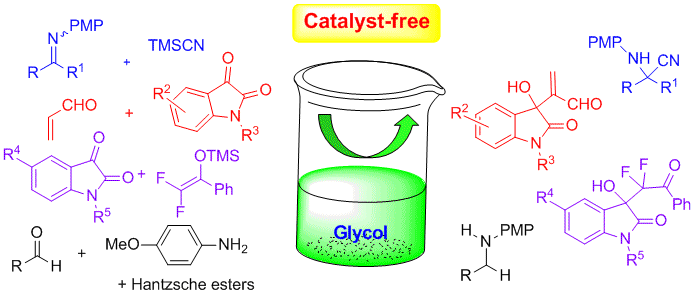
We report the use of ethylene glycol as a remarkable catalyst-free medium to accomplish a highly efficient Strecker reaction of α-CF2H or CF3 ketoimines and TMSCN‚ and the activation potency of ethylene glycol could be utilized for several reactions‚ including the first example of catalyst-free Morita-Baylis-Hillman reaction.
14) C.-B. Ji‚ Y.-L. Liu‚ X.-L. Zhao‚ Y.-L. Guo‚ H.-Y. Wang‚ J. Zhou*. Direct amination of α-substituted nitroacetates using di-tert-butyl azodicarboxylate catalyzed by Hatakeyama’s catalyst β-ICDOrg. Biomol. Chem.2012‚ 10‚ 1158-1161. (Invited for themed issue for Organocatalysis)13) F. Zhou‚ Z.-Y. Cao‚ J. Zhang‚ H. B. Yang‚ J. Zhou‚* A Highly Efficient Friedel–Crafts Reaction of 3-Hydroxyoxindoles and Aromatic Compounds to 3‚3-Diaryl and 3-Alkyl-3-aryloxindoles Catalyzed by Hg(ClO4)2⋅3H2O Chem. Asian J.2012‚ 7‚ 233-241. 12) Z.-Y. Cao‚ Y. Zhang‚ C.-B. Ji‚ J. Zhou‚*A Hg(ClO4)2·3H2O Catalyzed Sakurai–Hosomi Allylation of Isatins and Isatin Ketoimines Using Allyltrimethylsilane. Org. Lett.2011‚ 13‚ 6398-6401. 11) C.-B. Ji‚ Y.-L. Liu‚ Z.-Y. Cao‚ Y.-Y. Zhang‚ J. Zhou* Hydroxymethylation of α-substituted nitroacetates. Tetrahedron Lett. 2011‚ 52‚ 6118-6121. 10)F. Zhou‚ M. Ding‚ Y.-L. Liu‚a C.-H. Wang‚ C.-B. Ji‚ Y.-Y. Zhang; J. Zhou* Organocatalytic Asymmetric α-Amination of Unprotected 3-Aryl and 3-Aliphatic Substituted Oxindoles using Di-tert-butyl Azodicarboxylate (pages 2945–2952)Adv. Syn. Catal. 2011‚ 353‚ 2945-2952. 9) M. Ding‚ F. Zhou‚ Y.-L. Liu‚ C.-H. Wang‚ X.-L. Zhao‚ J. Zhou‚* Cinchona alkaloid-based phosphoramide catalyzed highly enantioselective Michael addition of unprotected 3-substituted oxindoles to nitroolefins. Chem. Sci.2011‚ 2‚ 2035-2039. (Highlighted by Synfact2011‚ 1245)8) Y.-L. Liu‚ T.-D. Shi‚ F. Zhou‚ X.-L. Zhao‚ X. Wang‚* J. Zhou‚* Organocatalytic Asymmetric Strecker Reaction of Di- and Trifluoromethyl Ketoimines. Remarkable Fluorine EffectOrg. Lett.2011‚ 13‚ 3826-3829. (Highlighted by Synfact 2011‚ 1015) 7)Y.-L. Liu‚ B.-L. Wang‚ J.-J. Cao‚ L. Chen‚ Y.-X. Zhang‚ C. Wang‚ J. Zhou‚* Organocatalytic Asymmetric Synthesis of Substituted 3-Hydroxy-2-oxindoles via Morita−Baylis−Hillman Reaction. J. Am. Chem. Soc. 2010‚ 132‚ 15176-15178.(Highlighted by Synfact 2010‚ 1422) 6) Y.-L. Liu‚ F. Zhou‚ J.-J. Cao‚ C.-B. Ji‚ M. Ding‚ J. Zhou‚* A facile method for the synthesis of oxindole based quaternary α-aminonitriles via the Strecker reactionOrg. Biomol. Chem.2010‚ 8‚ 3847-3850. (本文为封面文章) 5)J.-J. Cao‚ F. Zhou‚ J. Zhou‚* Improving the Atom Efficiency of the Wittig Reaction by a “Waste as Catalyst/Co-catalyst” Strategy (pages 4976–4980). Angew. Chem. Int. Ed.2010‚ 49‚ 4976-4980. 4)F. Zhou‚ Y.-L. Liu‚ J. Zhou‚* Catalytic Asymmetric Synthesis of Oxindoles Bearing a Tetrasubstituted Stereocenter at the C-3 Position (pages 1381–1407). Adv. Syn. Catal. 2010‚ 352‚ 1381-1407. 3)M. Ding‚ F. Zhou‚ Z.-Q. Qian‚ J. Zhou‚* Organocatalytic Michael addition of unprotected 3-substituted oxindoles to nitroolefins. Org. Biomol. Chem.2010‚ 8‚ 2912-1914. 2) J. Zhou‚* Recent Advances in Multicatalyst Promoted Asymmetric Tandem Reactions (pages 422–434)Chem. Asian J.2010‚ 5‚422-434. 1) Z.-Q. Qian‚ F. Zhou‚ T.-P. Du‚ M. Ding‚ B.-L. Wang‚ X.-L. Zhao‚ J. Zhou‚* Asymmetric construction of quaternary stereocenters by direct organocatalytic amination of 3-substituted oxindolesChem. Commun.2009‚ 6753-6755. Publications before independent work 1) J. Zhou‚ V. Wakchaure‚ P. Krafft‚ B. List‚ Primary Amine-Catalyzed Enantioselective Intramolecular Adolizations. Angew. Chem. Int. Ed.2008‚ 47‚ 7656-7658. (feature article‚VIP paper)。 2) J. Zhou‚ B. List‚ Organocatalytic Asymmetric Reaction Cascade to Substituted Cyclohexylamines. J. Am. Chem. Soc. 2007‚ 129‚ 7498-7499。 (本文入选2007年JACS第二季度“most accessed articles”) 3) J. Zhou‚ Y. Tang‚ Sidearm Effect: Improvement of the Enantiomeric Excess in the Asymmetric Michael Addition of Indoles to Alkylidene Malonates. J. Am. Chem. Soc.2002‚ 124‚ 9030-9031. 4) J. Zhou‚ Y. Tang‚ The development and application of chiral trisoxazolines in asymmetric catalysis and molecular recognition. Chem. Soc. Rev. 2005‚ 34‚ 664-676. (本文为封面文章). 5) L.-W. Ye‚ J. Zhou‚ Y. Tang‚ Phosphine-triggered synthesis of functionalized cyclic compounds. Chem. Soc. Rev. 2008‚ 37‚ 1140-1152. 6)S. C. Pan‚ J. Zhou‚ B. List‚ Catalytic Asymmetric Acylcyanation of Imines. Angew. Chem. Int. Ed. 2007‚ 45‚ 612-614. 7)W. Schrader‚ P. P. Handayani‚ J. Zhou‚ B. List‚ Characterization of Key Intermediates in a Complex Organocatalytic Cascade Reaction Using Mass Spectrometry. Angew. Chem. Int. Ed.2009‚ 48‚ 1463-1466. 8) V. Wakchaure‚ J. Zhou‚ S. Hoffmann‚ B. List‚ Catalytic Asymmetric Reductive Amination of a-Branched Ketones. Angew. Chem. Int. Ed.2010‚ 49‚ 4612-4614. 9) J.Zhou‚ Y. Tang‚ Enantioselective Friedel–Crafts reaction of indoles with arylidene malonates catalyzed by iPr-bisoxazoline–Cu(OTf)2.Chem. Commun.2004‚ 432-433. 10) J. Zhou‚ M.-C. Ye‚ Z.-Z. Huang‚ Y. Tang‚ Controllable Enantioselective Friedel-Crafts Reaction between Indoles and Alkylidene Malonates Catalyzed by Pseudo-C3-Symmetric Trisoxazoline Copper (II) Complexes. J. Org. Chem.2004‚ 69‚ 1309-1320. 11) J. Zhou‚ M.-C. Ye‚ Y. Tang‚ Sidearm Approach: A Promising Strategy for Construction of Bisoxazoline-Based Ligand Library. J. Comb. Chem.2004‚ 6‚ 301-304. 12) J. Zhou‚ Y. Tang‚ Pseudo-C3-symmetric trisoxazolines as ligands in copper catalyzed enantioselective Diels–Alder reaction. Org. Biomol. Chem.2004‚ 2‚ 429-433. 13) J. Zhou‚ B. List‚ Synthesis of trans-3-Substituted Cyclohexylamines via Brønsted Acid Catalyzed and Substrate-Mediated Triple Organocatalytic Cascade Reaction. Synlett2007‚ 2037-2040. 14) M.-C. Ye‚ J. Zhou‚ Z.-Z. Huang‚ Y. Tang‚ Chiral tris(oxazoline)/Cu(II) catalyzed coupling of terminal alkynes and nitrones. Chem. Commun.2003‚ 2554-2555. [被Angew. Chem. Int. Ed. 的Highlights 栏目评述 (Angew. Chem. Int. Ed.2004‚ 43‚ 2198.)]。 15) M.-C. Ye‚ J. Zhou‚ Y. Tang‚ Trisoxazoline/Cu(II)-Promoted Kinugasa Reaction Enantioselective Synthesis of Lactams. J. Org. Chem.2006‚ 71‚ 3576-3582. 16) S. C. Pan‚ J. Zhou‚ B. List‚ Catalytic Acylcyanation of Imines with Acetylcyanide. Synlett2006‚ 3275-3276. 17) Z.-Z. Huang‚ Y. B. Kang‚ J. Zhou‚ M.-C. Ye‚ Y. Tang‚ Diastereoselectivity-Switchable and Highly Enantioselective 1‚3-Dipolar Cycloaddition of Nitrones to Alkylidene Malonate. Org. Lett.2004‚ 6‚ 1677-1679‚ 18) M.-C. Ye‚ B. Li‚ J. Zhou‚ X.-L. Sun‚ Y. Tang‚ Modular Synthesis of Chiral Homo- and Heterotrisoxazolines.Improving the Enantioselectivity in the Asymmetric Michael Addition of Indole to Benzylidene Malonate. J. Org. Chem. 2005‚ 70‚ 6108-6110. (本文被Synfact收录‚ Synfacts‚ 2005‚ 203)。 19) J.-P. Qiu‚ Z.-H. Xu‚ J. Zhou‚ C.-L. Cao‚ X.-L. Sun‚ L.-X. Dai‚ Y. Tang‚ Ligand-Accelerated Asymmetric [1‚2]-Stevens Rearrangment of Sulfur Ylides via Decomposition o
Honor |

|
Zhoujian |